1. Background
Aging is associated with biological changes and the reduced life expectancy and adaptability of individuals to sudden changes. A gradual decline in the cellular and systemic function is also associated with increased age (1). In women, the ovary function slowly decreases with age, and the hormone production stops over several years of aging, which may further degrade after menopause (2). Some of the physiological changes caused by aging include changes in the body, lack of mobility, reduced physical fitness, changes in sex hormones or steroids, and conditions such as menopause, which are among the influential factors in insulin function disorders, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD) in the geriatric population (1, 3).
As a global epidemic, NAFLD is prevalent in 22% - 29% of the general population (4). In NAFLD, triglycerides (TGs) accumulate in the liver cells of a patient with no history of alcohol abuse (5). Several studies have indicated that NAFLD could increase the risk of cardiovascular diseases, diabetes, and mortality (6, 7). In order to prevent or delay the NAFLD onset and limit its consequences, the modification of NAFLD risk factors is considered essential.
Undoubtedly, exercise and physical activity (PA) play a key role in weight loss and may potentially contribute to reducing the levels of fatty liver (8). Some studies have shown the association of these disorders and NAFLD with low PA (9, 10). For instance, Kawanishi et al. (11) have reported an improvement in the indicators of liver damage in the rats with NAFLD after 16 weeks of aerobic training (11). Presumably, increased PA effectively prevents NAFLD by exerting protective effects against the development of type II diabetes, central obesity, hypertension, and dyslipidemia (8, 11).
Although several studies have examined the correlations between physical activity level (PAL), plasma lipids, and lipoproteins, few studies have been focused on the association of PAL and NAFLD despite its higher significance in elderly women.
2. Objectives
The present study aimed to investigate the association between PAL and NAFLD risk factors in the elderly women with NAFLD.
3. Methods
3.1. Participants and Study Design
The target population of the present study included the elderly women (age range: 60 - 65 years) who were diagnosed with NAFLD in Kermanshah, Iran. In total, 90 patients with NAFLD were selected via cluster sampling, and 42 women were enrolled in the study, and the remaining cases were excluded based on the exclusion criteria. Before the baseline procedures, two patients withdrew from the study.
The study protocol was approved by the Ethics Committee of Kermanshah University of Medical Sciences (trial code: IRCT20190423043359N1). Written informed consent was obtained from all the participants; in addition, participation in the study was voluntary, and the patients were allowed to withdraw from the study at any given time.
Before the training program, data were collected using the rapid assessment of physical activity (RAPA) questionnaire, which is a nine-item scale with yes/no responses and covers the levels of PA from sedentary to regular vigorous with strength and flexibility items that are scored separately. The instructions include the graphic and textual depictions of various activities and their categories. The total score of the first seven items is calculated within the range of 1 - 7 (1 = sedentary, 2 = underactive, 3 = irregularly underactive [light activities], 4 = irregularly active, 5 = regularly active + strength training = 1, flexibility = 2 or both = 3).
3.2. Body Composition and Anthropometric Measurements
Three days before and after the intervention, the study procedure was explained to the participants, and anthropometric parameters and body composition were measured. Height and waist circumference were measured to the nearest 0.5 centimeter using a stadiometer (DETECTO, model: 3PHTROD-WM, USA) and a non-elastic tape measure, respectively. To measure the body fat percentage (BFP), body mass index (BMI), and body weight (BW), the InBody test was performed using bioelectric impedance analysis (Zeus 9.9 PLUS; Jawon Medical Co., Ltd., Kungsang Bukdo, South Korea). Body composition measurements were performed early in the morning (8 AM - 9 AM) after a minimum of 12 hours of fasting overnight and emptying the bladder. The subjects were asked not to participate in intense PA and use no diuretic drugs within 48 hours before the measurements.
A standard manual sphygmomanometer was used to measure the systolic blood pressure (SBP) and diastolic blood pressure (DBP). The consumption of caffeinated products and exercising 30 minutes before the BP measurements were strictly prohibited. After relaxing in a seated state for 10 minutes, blood pressure was recorded twice at 8.00 AM - 10.00 AM in accordance with the standard guidelines of the American Heart Association. Finally, an average of two stable measurements was obtained (12).
3.3. Measurement of the Biochemical Variables
The subjects performed no exercises within 48 hours before the experiments and were fasting for 12 hours. Following that, 10 milliliters of blood was collected from the cubital vein of the patients for the enzymatic analysis of the blood lipid profile (Hitachi kit, Tokyo, Japan), liver enzymes (ELISA, Greiner Bio-One kit, made in Germany), insulin levels (ELISA, Mercodia kit, made in Sweden), and fasting glucose (Pars Azmun kit, made in Iran) in accordance with standard laboratory procedures. In addition, the insulin resistance index was performed using the homeostasis model assessment of insulin resistance (HOMA-IR) equation (6, 7), as follows:
The posttest was carried out 48 hours after the last training session. The degree of fatty liver (I, II or III) was reported by the results of ultrasonography (Color Doppler ultrasound, Siemens model, made in Germany), which was performed by a radiologist on the abdominal region of all the patients with the same equipment after a minimum of four hours of fasting at the imaging center of Imam Hossein Hospital.
3.4. Statistical Analysis
Data analysis was performed in SPSS version 21 (SPSS Inc., Chicago, IL, USA) at the significance level of P < 0.05. The Shapiro-Wilk test was used to evaluate the normality of data distribution, along with Pearson’s correlation-coefficient.
4. Results
Table 1 shows the mean values of the anthropometric indices, biochemical variables, and PAL of the patients. According to the findings, the patients had abnormal anthropometric indices and biochemical variables as diagnosed based on the clustering risk factor for NAFLD. The results also confirmed obesity in the examined patients.
| Variables | Values |
|---|---|
| Age, y | 62.25 ± 1.97 |
| Height, cm | 158.60 ± 5.19 |
| BW, kg | 86.70 ± 3.81 |
| BMI, kg/m2 | 34.54 ± 2.38 |
| Body fat percentage, % | 42.57 ± 3.42 |
| WHR, cm | 93.30 ± 3.03 |
| TG, mg/dL | 181.60 ± 9.93 |
| TC, mg/dL | 220.65 ± 10.26 |
| LDL, mg/dL | 140.07 ± 7.24 |
| HDL, mg/dL | 31.82 ± 5.38 |
| Glucose, mmHg | 134.45 ± 5.51 |
| Insulin, mmHg | 5.37 ± 0.83 |
| HOMA-IR | 1.78 ± 0.28 |
| SBP, mmHg | 129.55 ± 1.93 |
| DBP, mmHg | 84.02 ± 1.29 |
| ALT, U/L | 25.83 ± 3.27 |
| AST, U/L | 17.64 ± 1.03 |
| GGT, U/L | 36.07 ± 2.83 |
| Fatty liver (grade) | 2.35 ± 0.62 |
| Total PAL score | 1.70 ± 0.82 |
Abbreviation: ALT, alanine transaminase; AST, aspartate transaminase; BW, body weight; TG, triglyceride; DBP, diastolic blood pressure; GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein; SBP, systolic blood pressure; TC, total cholesterol; WHR, waist-to-hip ratio.
aValues are expressed as mean ± SD.
Table 2 shows significant, inverse correlations between all the studied variables and the PAL, except for HDL, which was positively correlated with the PAL (r = 0.495; P = 0.001). Among the variables, aspartate transaminase (r = -0.521; P = 0.001), HOMA (r = -0.505; P = 0.001), low-density lipoprotein (LDL; r = -0.497; P = 0.001), high-density lipoprotein (HDL; r = 0.495; P = 0.001), and total cholesterol (TC; r = -0.493; P = 0.001) were also significantly correlated with the PAL. On the other hand, BMI (r = -0.335; P = 0.035) had a less significant association with the PAL compared to the mentioned variables.
| NAFLD Risk Factors | Total PAL Score | |
|---|---|---|
| r | P Valuea | |
| BW, kg | -0.467 | 0.002 |
| BMI, kg/m2 | -0.335 | 0.035 |
| Body fat percentage, % | -0.472 | 0.002 |
| WHR, cm | -0.449 | 0.004 |
| TG, mg/dL | -0.486 | 0.001 |
| TC, mg/dL | -0.493 | 0.001 |
| LDL, mg/dL | -0.497 | 0.001 |
| HDL, mg/dL | 0.495 | 0.001 |
| Glucose, mmHg | -0.482 | 0.002 |
| Insulin, mmHg | -0.490 | 0.001 |
| HOMA | -0.505 | 0.001 |
| SBP, mmHg | -0.474 | 0.002 |
| DBP, mmHg | -0.465 | 0.002 |
| ALT, U/L | -0.481 | 0.002 |
| AST, U/L | -0.521 | 0.001 |
| GGT, U/L | -0.472 | 0.002 |
aSignificantly correlated with PAL; values calculated using Pearson’s correlation-coefficient.
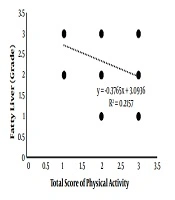
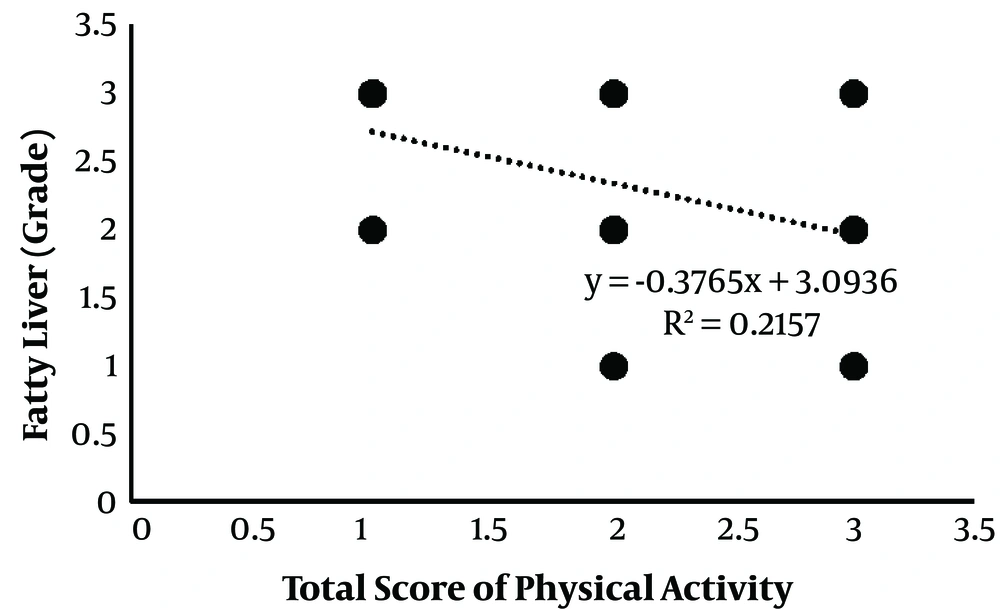
As is depicted in Figure 1, fatty liver grade and PAL were negatively correlated (r = -0.464; P = 0.003) as the higher activity of the individual was associated with the lower fatty liver grade.
5. Discussion
The present study aimed to investigate the correlation between PAL and NAFLD risk factors in Iranian elderly women with NAFLD, and the findings indicated significant, inverse correlations between PAL, anthropometric indices, and NAFLD risk factors in the elderly women with vitamin D deficiency. In addition, the RAPA questionnaire results, which have been reported to be valid in this age group (12), showed that none of the participants in the present study had high PAL.
In the current research, significant correlations were observed between the high levels of LDL, TG, TC, SBP, DBP, insulin, glucose, and HOMA-IR with low PAL. In addition, high HDL had a positive association with the PAL. As mentioned earlier, PA has a significant, inverse correlation with liver enzymes; therefore, observing an inverse correlation between the PAL and NAFLD in the present study was justified. A similar association between PAL and metabolic risk factors has also been reported in previous studies (13). A population‐based study in this regard has demonstrated significant, inverse correlations between PA and subscapular skinfold thickness, BMI, and waist circumference, which is consistent with the results of the present study (10). Furthermore, a significant association between PAL and insulin sensitivity has been observed in individuals with hypertension, which confirms the role of PA in metabolic risk factors (14).
According to a Norwegian study, physical fitness was a predictor of mortality in healthy, middle-aged individuals, and the correlations between metabolic risks and PA were attributable to the reduction of fasting insulin and TGs (15). Similar observations have also been reported by Wefers et al. (16), denoting that physical fitness was inversely correlated with insulin sensitivity, BMI, and blood pressure, while high PAL was associated with higher HDL. Observations in elderly women have also signified the efficiency of adequate PAL in improving cardiovascular factors after an eight-week exercise program (17). Other interventional studies on elderly women receiving physical training for eight weeks have indicated a significant reduction in the BW, BMI, body fat percentage, and WHR (18). Compensatory effects such as favorable changes in the TG level, total HDL cholesterol ratio, and DBP have also been demonstrated in adolescents (19).
In addition to the limitation in the inference about the direction of causality due to the cross-sectional design of our study, the improvements in the metabolic risk profile induced by PA were considered biologically acceptable, and the possible mechanism might be the enhancement of insulin action and glucose transport, which had been previously impaired in the elderly women (20, 21). Moreover, higher HDL cholesterol levels, lower blood pressure, and improved fat metabolism might be induced by increased capillarization and oxygen supply (higher blood flow) to the skeletal muscles after the PA adaptation in the elderly women (20, 22, 23). Increased PAL could also lead to higher lipoprotein A and the lipoprotein lipase (LPL) enzyme, which causes the lipid sector to catabolize, with the expected outcome of LDL reduction (24, 25). The increased activity of the LPL enzyme through plasma TG hydrolysis leads to protective effects against liver diseases (26, 27).
5.1. Conclusions
According to the results, NAFLD risk factors were inversely correlated with PA in the elderly women. Therefore, a physically active lifestyle is recommended to improve the metabolic risk factors in the elderly women with NAFLD who are less fit.