1. Background
Global research on diseases indicates that approximately 17.3 million deaths occur each year due to cardiovascular diseases (CVDs) (1). Chronic inflammations are a major cause of CVDs (2), and inflammatory markers are considered to be a strong predictor of CVDs (3). Obesity and overweight are a grave health concern worldwide, and the body fat percentage (BF%) of more than 25% and 32% in men and women, respectively is regarded a major risk factor in this regard (4) Excessive visceral fat cells increase the production of inflammatory proteins, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which are the mediators of obesity-related diseases, particularly CVDs (5). IL-6 is produced as a myokine during exercise and has anti-inflammatory effects, which are exerted through anti-inflammatory cytokines such as interleukin-10 (IL-10); IL-10 inhibits TNF-α (6).
Physical activity could be effective in the prevention and treatment of obesity and CVDs and improve body composition through anti-inflammatory mechanism (7, 8). Spinning exercise is a popular aerobic activity, which is performed in groups and with music and the guidance of a trainer. Since spinning is a recreational physical activity, it could encourage exercising and is effective in enhancing the cardiovascular system (9). As a result, training would be possible at high intensities (10) to reduce cardiovascular risk factors (11).
Today, special attention is paid to the use of herbal supplements for the prevention and treatment of CVDs. Green tea has remarkable anti-inflammatory effects, especially epigallocatechin-3-gallate in green tea weakens inflammatory responses (12). Recent findings have indicated that low-dose green tea supplementation has no influence on inflammatory and oxidative stress indices when provided along with resistance training (13). However, no research has been focused on the effects of spinning cycling and green tea on inflammatory markers. Systemic inflammation is associated with obesity, and spinning workout has a significant effect on body composition improvement (14) with a possible higher effectiveness within a shorter period. Therefore, the question of whether spinning workout and green tea supplementation have a significant effect on cardiovascular risk factors will be addressed in this paper.
2. Objectives
The present study aimed to investigate the effects of eight weeks of spinning workout and green tea supplementation on the anti-inflammatory and inflammatory markers and body composition of overweight women.
3. Methods
This study was conducted with a pretest- posttest and placebo-controlled design. Initially, the call to potential subjects was placed in all the sports clubs in Kermanshah city, Iran. Based on the inclusion criteria, 36 eligible young female volunteers were selected via simple random sampling. The inclusion criteria were as follows: (1) body mass index (BMI) of 25 ≤ BMI < 29.9 kg.m2 (15); (2) no prior experience of regular exercise for a minimum of six months before the study; (3) no specific diseases such as diabetes or CVDs; and (4) no use of anti-inflammatory supplements and special medications.
Prior to enrollment, the research objectives, possible risks, and applied methods were explained to the subjects, and written informed consent was obtained for participation. The study protocol was approved by the Institutional Review Board of Islamic Azad University, branch Sanandaj (code: IR.IAU.SDJ.REC.1399.015) and carried out in accordance with the latest version of the declaration of Helsinki (2013). At the next stage, the subjects were allocated to the groups of spinning workout-green tea (SP-GT), spinning workout-placebo (SP-PL), and control (no exercise/placebo) using a double-blind approach. In addition, the sample size was determined based on the previous studies (16, 17).
The exclusion criteria of the present study were absence in three consecutive training sessions and unwillingness to continue participation. Correspondingly, two subjects from the SP-GT and SP-PL groups were excluded due to not using the supplement and knee pain, respectively. In addition, two subjects were excluded from the control group due to unwillingness to continue participation. Finally, 32 subjects completed the study.
One week before the study, the subjects were invited to attend a fitness club to become familiar with the method and required measurements of the research. In order to understand the intensity of training based on the RPE, each subject was provided with a written document explaining the necessary instructions for the use to the Borg scale (6 - 20) (18). The SP-GT group used a green tea capsule three times daily (450 mg; Gia Essans, Co. Iran) after the main meals, and the placebo group consumed capsules containing dextrin at similar times (Zar Fructose, Co. Iran) with the same shape and taste as the green tea capsule. To prepare the placebo, 450 milligrams of dextrin powder was capsulized.
To measure anthropometric indices of the subjects (bodyweight, height, BMI, and BF%), bioelectrical impedance analysis was performed using the 720 InBody analyzer (Biospace Co., South Korea). To this end, the subjects stood on the device with minimal clothing and an empty bladder half an hour before testing. Following that, the anthropometric data were printed and recorded on a sheet (19).
Blood samples (10 ml) were collected from the cubital vein 48 hours before and after the first and last training sessions. To measure IL-10, the human serum kit was employed (ZellBio Co.Germany), and IL-6 and TNF-α were measured using the ELISA assay (Eastbiopharm Co., China). The blood samples were centrifuged at 3,000 rpm for 10 minutes and preserved at the temperature of -80 °C until the final measurements.
In order to evaluate the nutritional status of the subjects, a 24-hour dietary recall was performed 48 hours prior to both the blood sampling stages. The participants completed the 24-hour dietary recall form (all consumed foods, beverages, snacks, and fruits) 24 hours before and after the first and last blood sampling. Each food item was individually analyzed by Diet Analysis Plus (Cengage Co., USA) to measure the total consumed energy and macronutrients (carbohydrates, protein, and fat) (20).
Training was performed on 102 Fw spinning (Spin Bike Co. China) (21). The subjects received a training program three sessions per week, and the duration of each session was 45 minutes in the first two weeks, and five minutes were added to the sessions every two weeks. The training sessions were performed in groups with music and the guidance and encouragement of a trainer. Notably, no regular training program was assigned to the control group during the study period.
3.1. Statistical Analysis
Data analysis was performed in SPSS version 24, and the normality of data was determined using the Shapiro-Wilk test. Two-way repeated measures analysis of variance (ANOVA) was used to assess the intragroup and intergroup changes, and paired sample t-test was applied to measure the significance of the effect of time. In addition, the least significant difference (LSD) follow-up test was used to determine the significance of the group effect. In all the statistical analyses, the P-value of ≤ 0.05 was considered significant.
4. Results
Table 1 shows the anthropometric indices, body composition, and nutritional status of the subjects at the pretest and posttest. Accordingly, body weight and BMI significantly decreased in the training groups compared to the pretest. On the other hand, no significant differences were observed in the macronutrient and calories intakes of the study groups before and after the intervention.
| Variables/Groups | Pretest | Posttest | P-Value |
|---|---|---|---|
| Age (y) | |||
| SP-GT | 25.4 ± 3.5 | - | - |
| SP-PL | 24.8 ± 3.7 | - | - |
| Control | 24.3 ± 3.8 | - | - |
| Body Weight (kg) | |||
| SP-GT | 70.5 ± 7.8 | 67.5 ± 7.4c | 0.001c |
| SP-PL | 69.7 ± 4.7 | 67.7 ± 4.4c | 0.001c |
| Control | 69.5 ± 5.9 | 69.8 ± 6 | 0.06 |
| Height (cm) | |||
| SP-GT | 162.2 ± 4.7 | - | - |
| SP-PL | 161.9 ± 3.8 | - | - |
| Control | 162.1 ± 4.5 | - | - |
| BMI (kg/m2) | |||
| SP-GT | 26.7 ± 1.7 | 25.5 ± 1.7c | 0.001c |
| SP-PL | 26.8 ± 1.4 | 26.06 ± 1.4c | 0.001c |
| Control | 26.4 ± 1.4 | 26.5 ± 1.4 | 0.07 |
| Energy (kcal/day) | |||
| SP-GT | 1907 ± 17.6 | 1898 ± 19.1 | 0.66 |
| SP-PL | 1916 ± 28.4 | 1912 ± 24.8 | 0.57 |
| Control | 1918 ± 12.3 | 1920 ± 16.7 | 0.39 |
| Protein (g/day) | |||
| SP-GT | 57.5 ± 2.9 | 57.7 ± 2.05 | 0.66 |
| SP-PL | 59.1 ± 1.2 | 58.7 ± 1.01 | 0.17 |
| Control | 57.1 ± 1.4 | 56.6 ± 1.4 | 0.37 |
| Fat (g/day) | |||
| SP-GT | 59.02 ± 0.62 | 59.03 ± 0.63 | 0.99 |
| SP-PL | 58.7 ± 0.51 | 58.6 ± 0.75 | 0.87 |
| Control | 58.5 ± 1 | 58.8 ± 0.94 | 0.06 |
| Carbohydrate (g/day) | |||
| SP-GT | 286.4 ± 1.9 | 284.2 ± 8.3 | 0.38 |
| SP-PL | 287.8 ± 2.6 | 287.5 ± 2.9 | 0.88 |
| Control | 290.7 ± 2.1 | 291 ± 2.5 | 0.87 |
Abbreviations: BMI, body mass index; SP-GT, spinning-green tea; SP-PL, spinning-placebo.
a Values are expressed as mean ± SD unless otherwise indicated.
b Significant difference at P ≤ 0.05.
c Significant difference with pretest.
Table 2 shows the mean value of the research variables before and after the training intervention.
| Variables/Groups | Pretesta | Posttesta | Repeated Measures ANOVA | Pretest-Posttest | Differences | ||
|---|---|---|---|---|---|---|---|
| Source | F | p | |||||
| TNF-α (ng/l) | |||||||
| SP-GT | 289.14 ± 164 | 194.4 ± 88.9 | Time | 8.2 | 0.008 | SP-GT | 0.012b |
| SP-PL | 307.7 ± 92.1 | 292.1 ± 92.4 | Group | 7.2 | 0.003 | SP-PL | 0.001b |
| Control | 422.4 ± 106.9 | 432.2 ± 96.6 | Time × Group | 7.3 | 0.003 | Control | 0.191 |
| IL-6 (ng/l) | |||||||
| SP-GT | 235.19 ± 105.6 | 225.24 ± 96.09 | Time | 0.078 | 0.783 | SP-GT | 0.182 |
| SP-PL | 283.69 ± 86.08 | 280.82 ± 84.4 | Group | 4.38 | 0.022 | SP-PL | 0.24 |
| Control | 329.23 ± 44.8 | 349.67 ± 47 | Time × Group | 15.97 | 0.001 | Control | 0.011b |
| IL-10 (ng/ml) | |||||||
| SP-GT | 55.4 ± 18.4 | 64 ± 18.8 | Time | 25 | 0.001 | SP-GT | 0.001b |
| SP-PL | 59.4 ± 17.3 | 61.6 ± 16.07 | Group | 1.85 | 0.177 | SP-PL | 0.104 |
| Control | 46.7 ± 13.7 | 47.3 ± 12.8 | Time × Group | 10.48 | 0.001 | Control | 0.722 |
| BMI (kg/m2) | |||||||
| SP-GT | 26.7 ± 1.7 | 25.5 ± 1.7 | Time | 157.6 | 0.001 | SP-GT | 0.001b |
| SP-PL | 26.8 ± 1.4 | 26.06 ± 1.4 | Group | 4.7 | 0.018 | SP-PL | 0.001b |
| Control | 26.4 ± 1.4 | 26.5 ± 1.4 | Time × Group | 69.6 | 0.001 | Control | 0.074 |
| BF (%) | |||||||
| SP-GT | 34.6 ± 4.5 | 31.2 ± 3.6 | Time | 108.3 | 0.001 | SP-GT | 0.001b |
| SP-PL | 36.7 ± 4.2 | 35.3 ± 3.9 | Group | 4.65 | 0.018 | SP-PL | 0.001b |
| Control | 38.7 ± 2.6 | 38.2 ± 2.7 | Time × Group | 46.4 | 0.001 | Control | 0.27 |
Abbreviations: TNF-α, tumor necrosis-alpha; IL-6, interleukin-6; IL-10, interleukine-10; BF, body fat.
a Values are presented as mean ± SD.
bSignificant difference with pretest.
According to the findings, the intragroup changes (time effect) were significant in terms of BF%, IL-10, BMI, and TNF-α, as well as time and group interactions (Table 2). BMI significantly decreased in both training groups, and the effect size was estimated at 0.94 and 0.93 in the SP-GT and SP-PL groups, respectively. Regarding BMI, BF%, TNF-α, and IL-6, our findings indicated significant intergroup changes (group effect).
In the SP-GT and SP-PL groups, a significant decrease was observed in BF% after the intervention (P = 0.001), and the effect size was estimated at 0.92 and 0.79, respectively. On the other hand, IL-10 increased significantly after eight weeks in the SP-GT group (P = 0.001), while no significant change was denoted in the SP-PL group (P = 0.104), and the effect size was determined to be 0.87 and 0.24, respectively.
According to the obtained results, TNF-α significantly decreased in the SP-GT (P = 0.012) and SP-PL groups (P = 0.001), with the effect sizes estimated at 0.48 and 0.65, respectively. In addition, IL-6 decreased in both training groups although not significantly (P > 0.05). Table 3 shows the differences between the groups before and after the intervention. Accordingly, IL-6 had a significant difference between the SP-GT and control groups (P = 0.007).
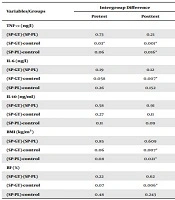
| Variables/Groups | Intergroup Difference | |
|---|---|---|
| Pretest | Posttest | |
| TNF-α (ng/l) | ||
| (SP-GT)-(SP-PL) | 0.73 | 0.21 |
| (SP-GT)-control | 0.03a | 0.001a |
| (SP-PL)-control | 0.06 | 0.016a |
| IL-6 (ng/l) | ||
| (SP-GT)-(SP-PL) | 0.19 | 0.12 |
| (SP-GT)-control | 0.058 | 0.007a |
| (SP-PL)-control | 0.26 | 0.152 |
| IL-10 (ng/ml) | ||
| (SP-GT)-(SP-PL) | 0.58 | 0.91 |
| (SP-GT)-control | 0.27 | 0.11 |
| (SP-PL)-control | 0.11 | 0.09 |
| BMI (kg/m2) | ||
| (SP-GT)-(SP-PL) | 0.85 | 0.609 |
| (SP-GT)-control | 0.06 | 0.007a |
| (SP-PL)-control | 0.08 | 0.021a |
| BF (%) | ||
| (SP-GT)-(SP-PL) | 0.22 | 0.62 |
| (SP-GT)-control | 0.07 | 0.006a |
| (SP-PL)-control | 0.48 | 0.243 |
a Significant difference at P ≤ 0.05.
According to the information in Table 3, the LSD post-hot test at the pretest indicated no significant differences between the groups in terms of BMI, BF%, IL-10, and IL-6 (P > 0.05), and only a significant difference was observed in the TNF-α between the SP-GT and control groups (P = 0.03). At the posttest, the intragroup difference in IL-10 was not considered significant (P > 0.05), and no significant difference was observed between the SP-GT and SP-PL groups in this regard.
According to our findings, the levels of TNF-α in the SP-GT (P = 0.001) and SP-PL groups (P = 0.016) decreased significantly compared to the control group. However, no significant difference was observed between the training groups in this regard. With regard to BF%, only a significant difference was denoted between the SP-GT and control groups (P = 0.006), while no significant difference was observed between the training groups. The mean changes in the BMI of the SP-GT (P = 0.007) and SP-PL groups (P = 0.021) also decreased significantly compared to the control group, and no significant difference was denoted between the training groups in this regard. Only the changes in IL-6 had a significant difference between the SP-GT and control groups (P = 0.007).
5. Discussion
In the present study, a significant reduction was observed in BMI and BF% after eight weeks of spinning workout and green tea consumption in both training groups compared to the pretest. Each unit of increase in the BMI is associated with the higher risk of CVDs by 8%, while each unit of MET (energy cost) (1 kcal.kg. hr-1) of physical activity decreases the risk of CVDs by the same percentage (22). In the present study, the reduction of BF% might be due to the reduced respiratory exchange ratio and increased lipolysis and utilization of fat substrates (23). Consistently, Afzalpour et al. (2015) and Ghasemi and Nayebifar (2019) reported a significant reduction in the BMI and BF% after 10 weeks of high-intensity interval training and green tea consumption (17, 24). The mechanism of the effects of green tea on BF% involves the inhibition of phospholipase A2 and acetyl-CoA carboxylase (25).
Other studies have reported the significant reduction of the BMI and BF% due to higher energy expenditure after spinning workout (14, 19, 21, 26). However, Moradi et al. (2016) observed a significant reduction in the BF% only in the group with green tea supplement and resistance training (27), which could be due to the fact that resistance training with green tea consumption improves body composition. Green tea also enhances the function of the sympathetic nervous system by inhibiting the enzyme catechol-o-methyltransferase, thereby increasing energy expenditure (28). It is possible that spinning workout increases fat oxidation due to higher energy expenditure, activating the AMP-activated protein kinase signal pathway (29). Another important mechanism of exercise in regards to BF% is the enhancement of proliferator-activated receptor coactivator 1-alpha, which plays a key role in metabolism control and prevention of obesity, especially visceral fat (17).
An important finding of the current research was that spinning workout without green tea consumption could improve body composition. After the eight-week intervention, IL-6 had a non-significant reduction in both training groups, while a significant increase was observed in the control group. Inactivity leads to the increased production of IL-6 from the fat cells in overweight individuals. At rest, IL-6 has a pro-inflammatory effect when released from the fat cells (10), while it exerts an anti-inflammatory effect when released from the muscle cells (e.g., IL-10) (7); notably, IL-10 inhibits the production of TNF-α (30). Inconsistent with the results of the present study, Bagheri et al. (2020) reported the significant reduction of IL-6 after endurance training and green tea supplementation (20). The discrepancy in this regard could be due to the differences in the training protocols, gender, and dose of supplementation.
According to the current research, the level of IL-10 increased after eight weeks of spinning workout and green tea consumption in both training groups, while it was only considered significant in the SP-GT group. The non-significant increase of IL-10 could be due to its inhibitory effect on TNF-α, which justifies the positive effect of spinning on the SP-PL group. On the other hand, a significant difference was denoted between the SP-PL and control groups in this regard, which could be attributed to the anti-inflammatory effects of spinning through the reduction of TNF-α at the posttest. The anti-inflammatory mechanism of physical exercise is the down-regulation of TNF-α and IL-6 gene expression and the up-regulation of IL-10 gene expression in macrophages (31). In a study, Barry et al. (2018) reported that short-term training had no effect on IL-10 (32), which is inconsistent with our findings possible due to the difference in the duration of the studies.
In the present study, a significant reduction was observed in TNF-α in both training groups. As a result, the anti-inflammatory effects of the spinning workout could improve TNF-α without green tea supplementation. In line with this finding, El-Kader and Al-Sheerf (2017) reported a significant decrease in TNF-α after resistance and aerobic exercises (8). However, the findings of Libardi et al. (2012) indicated no significant change in TNF-α after 16 weeks of resistance, endurance, and combination training. The discrepancy in this regard could be due to the lower TNF-α at baseline (33). TNF-α mediates local inflammation and is also involved in the systemic inflammation response. Moreover, this mediator plays a key role in the progression of CVDs through systemic inflammation (34). Recent findings have demonstrated no changes in TNF-α after endurance training along with the consumption of green tea (20, 35). It seems that the reduction of TNF-α is associated with the loss of body fat, which requires long-term training with high energy expenditure (e.g., spinning workout).
One of the limitations of the present study was the lack of a group to receive green tea alone as the intervention, however, green tea is widely known to improve body composition and systemic inflammation (23).
5.1. Conclusion
According to the results, spinning workout could reduce inflammation and improve the body composition of the overweight women. Furthermore, green tea increased the spinning-induced changes in IL-10. Since overweight women are at a high risk of CVDs, spinning may be a safe and cost-effective approach to weight loss within a short period, which could also help reduce inflammation and prevent CVDs. Finally, the costs of the rehabilitation and treatment of these patients and the mortality rate of CVDs would reduce if such interventions were implemented. Further investigations are recommended to clarify the physiological effects of spinning workout.