1. Background
The novel coronavirus is responsible for a widespread pandemic with millions of confirmed cases and a large number of deaths, which are reported in almost every country (1). COVOD-19 is caused by a novel coronavirus, which is mainly transmitted via droplets and contact surfaces. The virus is primarily considered to be a respiratory infection and is responsible for a clinical spectrum of manifestations, some of which show the subclinical course of the disease, while the others are associated with multisystem manifestations. In severe cases, the patients often present with progressive symptoms that start as a flu-like illness, followed by developing respiratory symptoms in the form of dry irritating cough, dyspnea, and increased inflammatory response to the viral infection, acute respiratory distress, multiorgan failure, and even death (2, 3).
The pathogenesis of the novel coronavirus remains unknown, and investigations are currently underway worldwide to find more information about the disease pathogenesis, as well as its clinical course, and long-term outcomes, and complications. A recent clinical reported the phenomenon of the reactivation of the disease in some patients after the initial clinical recovery, while data are still scarce in this regard (1, 4).
The disease is commonly diagnosed by a nasopharyngeal swab using the reverse transcriptase polymerase chain reaction (RT-PCR). However, this diagnostic test may yield high false negative rates. Chest CT-scan has shown extensive consolidative changes and extensive fibrosis mainly at the peripheral or bilateral patchy ground-glass appearance of the patients. Therefore, chest CT-scan appears to be more informative and specific in some patients. CT-angiography may also show associated vascular complications, such as thrombus formation. Nevertheless, no uniform approach is currently used for the diagnosis of COVID-19 in numerous regions (5, 6).
Peripheral blood films have shown changes in the morphology and count of white blood cells (WBCs), as well as platelet abnormalities. Furthermore, it is reported that the most common hematological abnormalities in these patients include low lymphocytes, increased neutrophils, reduced eosinophils, and changes in the platelets. Atypical and reactive lymphocytes may also be occasionally detected in the blood film, suggesting mild leukoerythroblastosis in some cases. The patients may also present with mild thrombocytopenia, while the severe reduction of the platelet count is quite rare (1, 3).
Reactive lymphocytes are frequently detected in many viral infections; such examples are infectious mononucleosis and dengue fever, both of which have various morphological characteristics. Some of the common subtypes that are detected in COVID‐19 patients are lymphocytes with a distinctive and abundant, pale blue cytoplasm, which often abuts adjacent the red blood cells. In addition, lymphoplasmacytoid lymphocytes may be observed in these patients, which consist of small and mature cells with a condensed chromatin and eccentric nucleus and a paranuclear hof in some cases. Lymphoplasmacytoid lymphocytes may be reported in patients with dengue fever and in some types of B‐cell non‐Hodgkin's lymphoma. The reactive lymphocytes of both types may coexist in the peripheral blood films of patients with COVID‐19 (7).
2. Objectives
The present study aimed to assess the changes in the blood smears of patients with COVID-19.
3. Methods
This study was conducted on 175 patients diagnosed with COVID‐19 infection. Data collection was performed at Azadi Teaching Hospital during August 25-September 15, 2020.
3.1. Blood Sampling
Under aseptic conditions, 2.5 milliliters of whole blood was collected in a K2EDTA tube, and the tube was placed on a mixer for five minutes.
3.2. Blood Smear Preparation
A drop of EDTA blood was placed in the central line of a clean glass slide at one centimeter of distance from one end. Afterwards, the drop was spread quickly with a spreader at an approximate angle of 30 degrees, and the slide was left to air-dry.
3.3. Staining Method
Blood films are prepared and left to dry at room temperature. The air-dried films were flooded with Leishman stain for two minutes, and distilled water was added with the doubled volume of the stain for 5 - 7 minutes. After washing in a stream of buffered water, the back of the slide was wiped clean and placed upright to dry.
3.4. WBC Differential Count
Blood films were inspected at low magnification (× 10 objective), and WBC differential count was performed manually after reading 100 cells at 40X power. In the cases with abnormal WBC counts, up to 200 cells were counted. WBC counts were expressed as the absolute value due to the utmost importance of the relative value and the fact that benign leucocyte definitions rely on the absolute count.
Leukocytes differentials were also carried out by counting up to 100 cells. In some cases, the normal individuals did not show eosinophils in the smear, and the definition of eosinophilia and/or eosinopenia may require counting up to 500 WBCs.
3.5. Reference Values
Anemia is defined as the reduced number of red blood cells or hemoglobin (Hb), which results in the decreased capability of the blood to carry oxygen to the tissues. Based on the World Health Organization (WHO) recommendations, anemia is diagnosed if the Hb level is < 12.0 g/dL in females (normal: 12 - 15 g/L) and < 13.0 g/L in males (normal: 13 - 17 g/L).
Leukocytosis is defined as the total WBC count of more than > 11 × 109/L, and leucopenia is defined as the total WBC count of less than 3 × 109/L. Neutropenia is defined as the total WBC count of more than 1.5 × 109/L, and neutrophilia is defined as the absolute neutrophil count of more than 7.5 × 19/L. Lymphopenia is defined as the absolute lymphocyte count of less than 1.0 × 109/L, and lymphocytosis is defined as the absolute lymphocyte count of more than 3.5 × 19/L. Monocytosis is defined as the absolute monocyte count of more than 1.0 × 1^9/l, and monocytopenia is defined as the absolute monocyte count of less than 0.2 × 19/L. The normal range of eosinophils is 0.02 - 0.5 × 119/L (1 - 6%), and eosinophilia refers to the count of > 0.5 × 19/L. Thrombocytopenia is defined as the platelet count of less than the lower limit of normal (< 150 × 109/L), and thrombocytosis refers to the higher platelet count than the upper limit of normal (> 450 × 109/L) (8-13).
4. Results
In total, 175 patients diagnosed with COVID-19 infection with the mean age of 53.73 years were enrolled in the study. Males constituted 52.6% of the sample population, and the majority had normal Hb levels (Table 1). WBC count was normal in 60.6% of the patients, and 74.3% had a normal platelet count.
| Variables (n = 175) | No. | % |
|---|---|---|
| Gender | ||
| Male | 92 | 52.6 |
| Female | 83 | 47.4 |
| Age (mean ± SD) (range: 17 - 87 y) | 53.73 | 14.569 |
| Hemoglobin | ||
| Normal | 139 | 79.4 |
| Low | 36 | 20.6 |
| MCV | ||
| Normocytic | 149 | 85.1 |
| Microcytic | 24 | 13.7 |
| Macrocytic | 2 | 1.1 |
| WBC | ||
| Normal | 106 | 60.6 |
| Leukocytosis | 56 | 32.0 |
| Leukopenia | 13 | 7.4 |
| Platelets | ||
| Normal | 130 | 74.3 |
| Thrombocytosis | 4 | 2.3 |
| Thrombocytopenia | 41 | 23.4 |
Abbreviations: MCV, mean corpuscular volume; WBC, white blood cell.
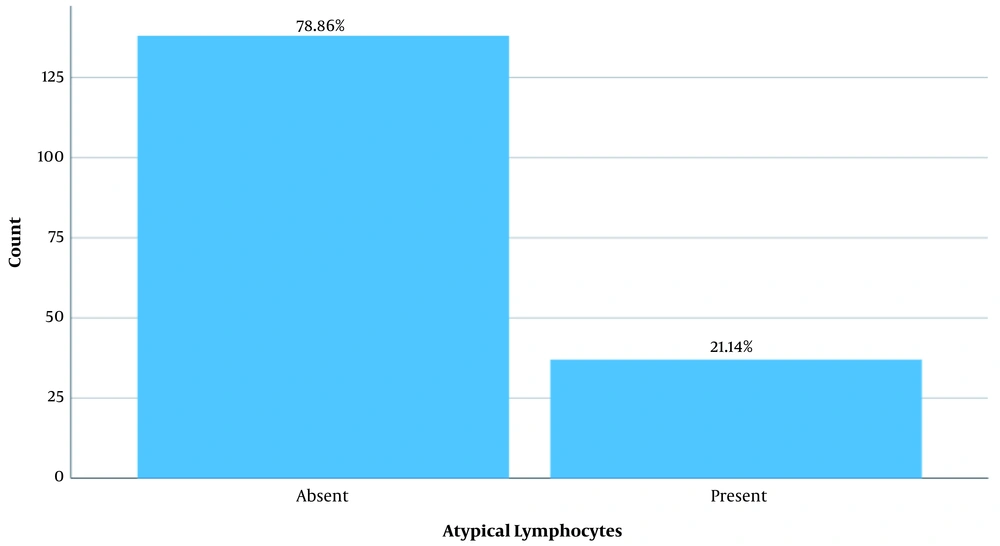
Differential WBC count showed that 56.6% of the patients had normal neutrophils, 62.9% had normal lymphocytes, 73.7% had normal monocytes, and 77.7% had a low eosinophil count (Table 2). The peripheral blood smear of the patients with COVID-19 infection showed atypical lymphocytes with deeply basophilic cytoplasm in 21.14% of the cases (Figure 1). In addition, the smear of some patients showed lymphocyte with spreading cytoplasm and lymphocytes with plasmacytoid features, which could be used for the diagnosis of COVID-19 (Figure 2).
| Variables | No. | % |
|---|---|---|
| Neutrophils | ||
| Normal | 99 | 56.6 |
| Neutrophilia | 67 | 38.3 |
| Neutropenia | 9 | 5.1 |
| Lymphocytes | ||
| Normal | 110 | 62.9 |
| Lymphocytosis | 16 | 9.1 |
| Lymphopenia | 49 | 28.0 |
| Monocytes | ||
| Normal | 129 | 73.7 |
| Monocytopenia | 21 | 12.0 |
| Monocytosis | 25 | 14.3 |
| Eosinophils | ||
| Normal | 35 | 20.0 |
| Eosinophilia | 4 | 2.3 |
| Low Eosinophil | 136 | 77.7 |
5. Discussion
The patients who are diagnosed with COVID-19 infection exhibit several hematological abnormalities, which are detected in the measurement of blood parameters, blood film examinations or bone marrow aspirates. Despite the clinical, biological, and hematological changes in the patients infected with the novel coronavirus, anemia has not reported as a predominant feature. In the present study, the Hb level was normal in 79.4% of the patients. Some studies have suggested anemia in these patients may be of an autoimmune origin although this has not been proved so far (1, 6).
The exact mechanism of decreased lymphocyte count in the peripheral blood remains unclear, while some of the possibilities include the mobilization of the cells into infection sites and the virus-induced destruction of T cells (14).
Neutrophil count has been reported to increase within a few days after the infection. In the current research, neutrophilia was observed in 38.3% of the patients, and neutropenia was detected in 5.1%. Neutrophil absolute count often increases in the early stages of the disease and may gradually decrease in the absence of septic complications or bone marrow suppression (3).
In the current research, the morphological changes in the peripheral blood smears included neutrophilia and lymphopenia with the presence of several abnormal shaped lymphocytes, such as atypical lymphocytes with deeply stained basophilic cytoplasm, lymphocytes with spreading cytoplasm, and lymphocytes with plasmacytoid features. Furthermore, the majority of the patients had a low eosinophil count. Platelet count also indicated changes, which were normal or reduced in some cases. Some studies have confirmed that the lymphocyte population may show wide-ranging morphological heterogeneity, such as the presence of large atypical lymphocytes, lymphoplasmacytoid cells, and a large proportion of outsized granular lymphocytes. Morphological abnormalities have also been reported to disappear after treatment and recovery after one week (1, 3, 15, 16).
During viral infection, B-lymphocytes become activated to form lymphoplasmacytoid lymphocytes and immunoglobulin-secreting plasma cells, which have a distinctive morphology. According to the findings in China, lymphopenia has been observed in approximately 85% of COVID-19 patients, while the rate of lymphopenia in our patients was 28%, which is consistent with another study in China. The difference in the rate of lymphocytes depends on several factors and different body responses to the pathogen. Therefore, further evaluations my show the reduced number of CD4-positive and CD8-positive T cells, B cells, and natural killer cells. A favorable clinical outcome has also been correlated with increased lymphocyte count, and lymphocytosis was present in 9.1% of the patients in the current research (1, 15, 17, 18).
Abnormal lymphocytes typically appear in the peripheral blood films within a few days after the onset of symptoms. Other characteristics of atypical lymphocytes include the presence of medium-sized or large cells with lightly condensed chromatin material and moderately/deeply stained basophilic cytoplasm. Some cells may also have a plasmacytoid appearance with an eccentric nucleus, while other cells may have one/more than one nucleus, which might be mistaken with blast cells. The detection of plasmacytoid lymphocytes in the peripheral blood film may support the diagnosis of COVID-19 infection (19).
According to a study conducted in France, sever thrombocytopenia (< 50 × 109/L) may be very unusual, while it is considered to be an independent risk factor for ICU admission. In the present study, 74.3% of the patients had a normal platelet count, and the rate of thrombocytopenia was estimated at 23.4%. Our findings in this regard are consistent with the worldwide data, indicating that mild thrombocytopenia is present in up to 20 ‐ 36% of the patients infected with COVID-19. The cause of thrombocytopenia is multifactorial, and it may be caused by the activation and aggregation of platelets as a result of a hypercoagulable state and microthrombi formation, which has been observed in some cases. Moreover, COVID-19 infection has been associated with the direct destruction of the hematopoietic cells and bone marrow stem cells, which in turn lead to hematopoietic dysfunction. A cytokine storm may also cause damage to the bone marrow progenitor cells, and elevated serum antibodies and immune complexes could increase platelet destruction (1, 19, 20).
5.1. Conclusion
Blood film examination is an important diagnostic tool for COVID-19 infection as it shows numerous abnormal findings. Neutrophilia and lymphopenia with the presence of atypical lymphocytes have been frequently detected in these patients. On the other hand, low eosinophil count is reported to be highly common. Despite changes in platelets, they are either normal or reduced in most cases.
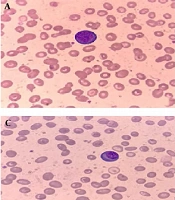
![Peripheral blood smear of patients with COVID-19 infection; A, atypical lymphocytes with deeply basophilic cytoplasm; B, neutrophils with abnormal segmentation [three neutrophils with bilobed nuclei (pseudo-Pelger-Huet nuclei)]; C-D, lymphocytes with plasmacytoid features. Peripheral blood smear of patients with COVID-19 infection; A, atypical lymphocytes with deeply basophilic cytoplasm; B, neutrophils with abnormal segmentation [three neutrophils with bilobed nuclei (pseudo-Pelger-Huet nuclei)]; C-D, lymphocytes with plasmacytoid features.](https://services.brieflands.com/cdn/serve/3170b/ccce4b14ad237cbbeaf3d9f0484433babddf4c4b/jkums-110758-g001-F2-preview.webp)