1. Background
Hyperlipidemic diabetes (HD) is one of the most common chronic diseases in the present century (1). Hyperlipidemic diabetes is characterized by an improper response to insulin, disruption of fat, carbohydrate, and protein metabolism, and eventually hyperglycemia (2). According to the latest data on diabetes incidence, there are 230 million cases of diabetes worldwide and 3 million cases in Iran (3, 4). Hyperlipidemic diabetes affects various aspects of individuals’ life causing physical and psychological problems by increasing the risk of comorbidities (5). The most significant predictors of HD are sedentary lifestyles, unhealthy diets, and reduced physical activity (6). A general concept called Quality of Life (QoL), which is impaired in patients with HD, can shed light on the overall health status of a person (7-9). The primary goal of HD management is achieving higher levels of QoL and preventing the onset of comorbidities (10). As a non-pharmacological solution, a regular exercise program has been known as a critical component of HD improvement to reduce the complications of diabetes and improve the mental status and QoL (11, 12). Exercise benefits QoL by increasing the average scores of psychosocial physical domains in patients with HD (13), among which aerobic training (AT) plays an essential role in increasing insulin-induced glucose uptake, strengthening skeletal muscles, reducing depression, and thus enhancing QoL (14, 15).
As a result of COVID-19 onset, individuals with chronic diseases such as diabetes are now at higher risk of developing viral infections (16, 17). Quarantine recommendations focusing on staying home impacted social behaviors and physical activity levels, leading to inactivity and reduced QoL (18). Aerobic training is an appropriate approach to reduce inactivity and increase QoL in COVID-19 susceptible populations (19).
Herbal supplement, such as turmeric supplementation (TS), is another strategy to control and prevent HD (20-22), which has been considered due to its lower side effects, availability, and cost-effectiveness (23). Due to the presence of curcumin, TS is known to have the most therapeutic effects on glycemic control and to reduce the complications of HD via antioxidant properties, which might also increase the QoL (24-26). Maithili Karpaga Selvi et al. reported that TS enhances blood glucose, oxidative stress, and inflammation in patients with T2DM (27). However, Adab et al. observed the improvement of bodyweight following TS in patients with HD and reported no significant effect on total antioxidant capacity, hs-CRP, and glycemic status (28). Although numerous studies have examined the effects of TS on the physiological status of diabetics, no study has examined the effects of TS on the quality of life of HD patients.
2. Objectives
Due to the limited results in the literature investigating the simultaneous effect of AT + TS, the purpose of the present study was to evaluate the interactive effect of AT + TS on the QoL during COVID-19 quarantine in type 2 diabetic women with hyperlipidemia.
3. Methods
3.1. Trial Design and Participants
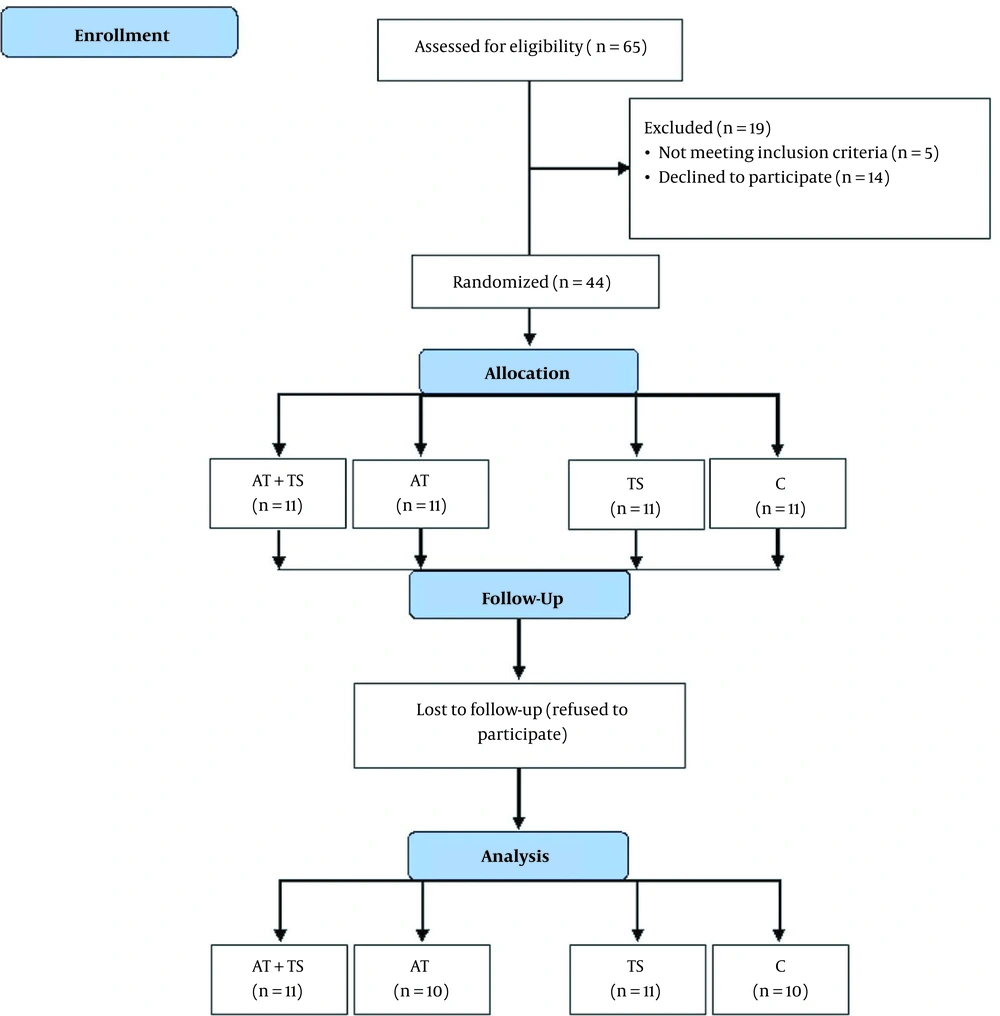
This quasi-experimental (single-blinded) study was conducted with three experimental groups and one control group. Forty-four women with type 2 diabetes were selected (The Diabetes Center of Taleghani Hospital, Kermanshah, Iran) by purposive and convenient sampling. In addition, G. POWER 3.1 was used at a significance level of 0.05 with a statistical power = 80 % and an effect size = 95%. Then, the subjects were randomly divided into four groups, including control + placebo (C; n = 11), AT + placebo (AT; n = 11), TS (n = 11), and AT + TS (n = 11). Two subjects refused to continue the study (one in AT and one in C; Figure 1).
The inclusion criteria included women diagnosed with type 2 diabetes, aged 40 to 65 years, and classified as overweight (body mass index (BMI), 25 to 29.99 kg/m2). The exclusion criteria included having regular exercise, a history of other diseases, smoking, and COVID-19 infection during the intervention.
The Ethics Committee of Kermanshah Razi University approved this trial (IR.RAZI.REC.1400.008). This study was also registered in the Iranian Clinical Trial Registration Center (IRCT20201129049524N1). All participants completed and signed a written informed consent to participate or leave the study voluntarily. The medical history questionnaire revealed no evidence of coronary artery disease, renal failure, or thyroid disease among the subjects. The information about research conduction, possible risks, the online home-based exercise sessions, and controlling the exercise intensity during the training sessions was explained to the subjects. In addition, the informed consent and physical activity readiness questionnaire were completed. Then, the initial variables, including height, weight, and body fat percentage, were measured.
3.2. Intervention
3.2.1. Aerobic Training
Aerobic training was conducted at home under the online supervision of exercise physiologists three times per week for eight weeks. The AT started with 20 min at 60% of HRmax, and increased to 40 min at 75% of HRmax according to the American Diabetes Association (ADA) (29) (Table 1). Warming up and cooling down time were each 10 minutes.
| Variables | Week | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Intensity (HRMax, %) | 60 - 65 | 60 - 65 | 60 - 65 | 65 - 70 | 65 - 70 | 65 - 70 | 70 - 75 | 70 - 75 |
| Time (min) | 20 | 25 | 30 | 30 | 35 | 35 | 40 | 40 |
| Borg scale | 10 | 10 | 11 | 11 | 12 | 12 | 13 | 13 |
The HRmax formula [HRmax = 220 - age] determined the target heart rate (30). The participants learned the pulse palpation method to count their pulse or heart rate in an instructional session. To assure achieving the desired exercise intensity, the rating of perceived exertion (RPE) (6 - 20 scale) was considered during the exercise session (31) (Table 1).
3.2.2. Diet and Turmeric Supplementation
The participants filled out two questionnaires to attend the training program. First, a questionnaire assessed demographic data, physical activity, and health status. Then, a detailed semi-quantitative food record questionnaire adapted to the Iranian population was used before and after the intervention that was composed of a limited checklist of foods and beverages (everyday food items, serving sizes, meals) with a separate frequency response section and portion size for subjects. Participants were requested to consume the same macronutrient composition one day before the post-test. The Food Processor Nutritionist Four (FPN4) software determined the food intake via multiplying the reported frequency of each food by the macronutrient amount (protein, fat, and carbohydrates) in a serving size. The diet consisted of 15% protein, 30% fat, and 55% carbohydrates. Table 2 shows the carbohydrate, protein, and fat consumption. The reliability and validity of this questionnaire are also evaluated.
| Groups | Carbohydrate (g) | Protein (g) | Fat (g) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| AT + TS | 387.8 ± 123.22 | 360.4 ± 138.13 | 112.3 ± 18.01 | 116.6 ± 22.04 | 95.15 ± 7.01 | 92.7 ± 8.02 |
| AT | 360.7 ± 133.41 | 347.7 ± 98.42 | 107.2 ± 23.08 | 112.62 ± 16.02 | 98.18 ± 6.03 | 96.14 ± 5.04 |
| TS | 383.3 ± 88.29 | 365.6 ± 117.18 | 115.35 ± 24.06 | 118.63 ± 13.03 | 100.16 ± 8.07 | 99.11 ± 4.03 |
| C | 357.8 ± 123.31 | 366.6 ± 89.23 | 110.3 ± 27.11 | 111.5 ± 11.07 | 88.14 ± 11.02 | 100.11 ± 8.01 |
Abbreviations: C, the control group; TS, turmeric supplement group; AT, aerobic training group; AT + TS, aerobic training + turmeric supplement.
According to previous research, consuming 1 - 3 g of turmeric per day is safe. Therefore, 2100 mg per day (2.1 g per day) was determined based to human studies (32). In the TS and AT + TS groups, 700 mg of turmeric powder was consumed as part of each meal for eight weeks. The AT and C received three capsules containing 700 mg of cornstarch flour, which were identical in shape, color, and packaging.
3.2.3. Anthropometric Measurements and Body Composition
Three days before the intervention, anthropometric parameters and body composition were measured. A stadiometer (DETECTO, Model 3PHTROD-WM, USA) was used to measure height to the nearest 0.5 cm. A non-elastic tape measure was utilized to determine waist circumference to the nearest 0.5 cm. In addition, the BMI and weight were determined using bioelectric impedance analysis by the INBODY test (Zeus 9.9 PLUS; Jawon Medical Co., Ltd., Kungsang Bukdo, South Korea) in the morning after at least 12 hours of fasting. Subjects were asked to refrain from taking diuretic drugs and diuretics 48 hours before the measurements.
3.3. Quality of Life
The SF-12 was used to evaluate QoL. It has two summary components of the mental component summary (MCS) and physical component summary (PCS), 12 questions, and eight scales, including mental health (MH), role limitations due to emotional problems (RE), general health perception (GHP), vitality (VT), social functioning (SF), physical functioning (PF), role limitations due to physical health (PH), and body pain (BP). The final score of the SF-12 ranged from 0 to 100, with a higher score indicating a better QoL. The SF-12 was adapted and validated to Persian conditions by Rohani et al. (33).
3.4. Data Analysis
The data were analyzed at a significant P < 0.05 using the SPSS (Version 21; SPSS Inc., Chicago, IL, USA) Software. To evaluate the normality of distribution, the Shapiro-Wilk’s test was used. The t-test and ANOVA were used to compare the mean variables within and between groups. In addition, Tukey’s post hoc test was used in the case of significant differences.
4. Results
The descriptive characteristics of the participants are presented in Table 3. As shown in Table 3, the mean ± SD of BMI and body fat percentage of all participants were in the overweight category. Furthermore, all subjects in the present study had the indicators of type 2 diabetes and hyperlipidemia.
| Variables | AT + TS | AT | TS | C | P-Value a |
|---|---|---|---|---|---|
| Age (y) | 43.02 ± 3.04 | 42.13 ± 2.39 | 44.33 ± 1.23 | 44.22 ± 3.07 | 0.336 |
| Height | 160.12 ± 4.11 | 163.13 ± 3.03 | 159.24 ± 2.79 | 161.13 ± 5.08 | 0.393 |
| Body weight (kg) | 73.12 ± 2.91 | 75.13 ± 2.07 | 74.13 ± 2.68 | 75.09 ± 3.20 | 0.061 |
| Body mass index (kg/m2) | 28.15 ± 1.02 | 28.12 ± 1.23 | 29.30 ± 0.30 | 29.02 ± 1.04 | 0.223 |
| Body fat percent (%) | 30.14 ± 1.26 | 32.09 ± 2.01 | 29.23 ± 1.27 | 31.16 ± 2.52 | 0.206 |
| WHR | 0.90 ± 0.03 | 0.92 ± 0.04 | 0.91 ± 0.05 | 0.92 ± 0.02 | 0.229 |
Abbreviations: C, the control group; TS, turmeric supplement group; AT, aerobic training group; AT + TS, aerobic training + turmeric supplement.
a P values is calculated using one-way analysis of variance test followed by post hoc Tukey’s test.
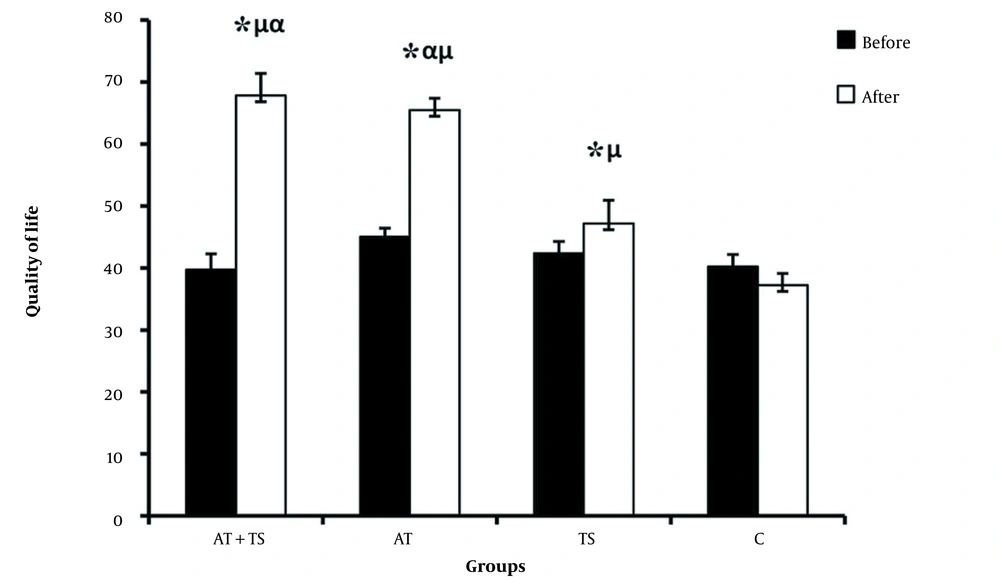
The correlated t-test shows that AT + TS, AT, and TS decreased the QoL in the post-test compared to the pre-test, which was not statistically significant. In addition, the C group showed decreased QoL (Figure 2). The results of one-way ANOVA showed no significant difference in the mean QoL in the pre-test between groups, while a significant difference was observed in the post-test. Tukey’s post hoc test results showed a significant difference in the QoL between the AT + TS, AT, and TS with C. Moreover, there was a significant difference between AT + TS with TS but not with AT. Besides, a significant difference was observed between the AT and TS.
Comparison between mean ± standard deviation of QoL between groups; C, the control group; TS, turmeric supplement group; AT, aerobic training group; AT + TS, aerobic training + turmeric supplement; *, significant differences comparing pre and post-test; μ significant differences compared to C; α, significant differences compared to TS
5. Discussion
Most of the evidence about the health benefits of physical activity is based on epidemiological studies, and there are very few interventional studies. However, our knowledge of the interactive effects of AT + TS on the QoL in women with HD is limited.
The present study indicated that eight weeks of AT + TS, AT, and TS significantly increased QoL. However, the interactive effect of AT + TS was higher than the separate effect. Our results are in line with Cai et al. (34), Chiang et al. (35), and Delevatti et al. (36), reporting improved QoL in patients with HD following separate AT or TS interventions. The positive effect of AT on improving the QoL in patients with HD might be probably due to the improved biochemical structure of muscle, increased oxygen consumption, and reduced insulin resistance (37). According to other studies, AT reduces the required proinsulin mRNA to synthesize insulin and decreases glucokinase levels (29, 30). This, in turn, decreases blood insulin levels and phosphorylated insulin receptors (38). Furthermore, AT activates the central nervous system and increases the secretion of endorphins, norepinephrine, serotonin synthesis, and endorphins, which reduces complications and improves QoL in patients with HD (39).
In addition, TS has been shown to have antioxidant, anti-inflammatory, antibacterial, antiviral, and anti-cancer characteristics with a potential role in treating many chronic diseases such as diabetes (40). Possible mechanisms by which turmeric improves the QoL in patients with HD include increased acyl-CoA cholesterol acyltransferase (ACAT) and carnitine palmitoyltransferase 1 (CPT1) (41, 42), decreased IL-6, HbA1c, and TNF-α (43, 44) inhibited MCP-1, and suppressed NFκB signaling pathway (45). Thus, AT + TS might ultimately improve QoL in HD patients through the mechanisms outlined above.
5.1. Conclusions
Eight weeks of separate and interactive AT and TS improved the QoL in women with HD. However, the mechanisms involved in these changes have not yet been fully elucidated. According to results, AT + TS might be an appropriate non-pharmacological treatment to improve the QoL in patients with HD, given the positive effects of AT + TS. The main limitations of this study included the small sample size and not controlling the food intake within the eight weeks. More accurate results could be achieved by more control.