1. Background
Since the mid-1900s, antibiotic therapy has been introduced for the treatment of various patients. However, antibiotic overuse has had debilitating effects on every aspect of the healthcare system; such examples are increased medication costs, poor patient management, and bacterial resistance (1). The misuse and overuse of antibiotics, which undermines a century of advancement in the discovery of antibiotics and the contribution of antibiotics to health improvement in developing countries (2), is a major influential factor in antibiotic resistance in humans and animals (3).
According to the Centers for Disease Control and Prevention (CDC), antibiotic resistance has led to the resistance of various infections to antibiotics in areas with the overuse of antibiotics. The CDC estimates that 30% of antibiotics are unnecessary (4). Published data are limited regarding the extent of this issue in low- and middle-income countries. However, high-level resistance is increasingly reported around the world (5). The American Society of Infectious Diseases (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) strongly believe that antibiotic monitoring programs must be carried out by infectious disease specialists with complementary trainings. An antibiotic monitoring program encompasses a wide range of activities, such as replacing the therapeutic regimens with cost-effective antibiotics of the same groups, switching from intravenous to oral medications, and using pharmaceutical advices to choose antibiotics (6).
Due to the misuse of antibiotics and the development of resistance to these agents, some countries have developed and promoted an antibiotic monitoring program known as stewardship. This program is based on a national plan and consists of international standard elements, including setting and implementing guidelines, training physicians, establishing networks for prescribing and monitoring antibiotics, and adopting policies to limit antibiotic prescription (2). The stewardship program describes a set of measures that could be taken for the proper use of antibiotics, including optimal evidence-based standards for the routine use of antibiotics (eg, correct choice of antibiotics, dosing, administration route, and treatment duration), ensuring training programs for individuals using antimicrobials, communicating antibiotic issues with stakeholders, assessing the effects of the plan, and optimizing the outcomes for the patients receiving antibiotics (7).
In Iran, the stewardship program was initially implemented by the Ministry of Health and Medical Education for nine antibiotics. The program was evaluated and implemented in Imam Reza Hospital in Kermanshah, which is a general hospital with 34 wards.
2. Objectives
Given the importance of antibiotics and their use in hospitalized patients, the present study aimed to compare the use of antibiotics before and after the implementation of the stewardship program.
3. Methods
This retrospective study aimed to compare the use of antibiotics during six months before and after the implementation of the stewardship program in patients admitted to Imam Reza Hospital in Kermanshah, Iran during December 2017 - December 2018 (December 2017 - May 2018: six months prior to the stewardship program, July 2018 - December 2018: six months after the implementation of the stewardship program). The research project started in June 2018 in Imam Reza Hospital. Prior to the implementation of the protocol, a training session was held for all the hospital specialists to demonstrate the protocol and ensure the recognition of its effective implementation.
The hospital quality control department ensured compliance with the protocol in the hospital departments with quarterly reports. Data of the patients were extracted from the electronic records available in the pharmaceutical ward of the hospital. The antibiotic groups mentioned in the patients’ files included imipenem, liposomal amphotericin B, teicoplanin, caspofungin, colistin, linezolid, meropenem, voriconazole, and vancomycin, which were classified and coded 1-9 by software.
The selected hospital has 34 wards, which were divided into the surgery ward, internal medicine ward, and intensive care unit (ICU) in terms of efficiency. The per capita numerical consumption of antibiotics and per capita costs (Rials) were also extracted from the data.
Data analysis was performed using an electronic database in SPSS version 21 using median and interquartile range (IQR) to describe the quantitative data. In addition, the Mann-Whitney U test was applied to analyze the data.
4. Results
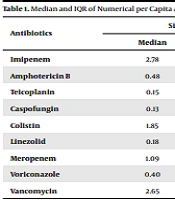
In total, the records of 677 patients prior to the implementation of the program and 637 patients after the implementation of the program were evaluated in Imam Reza Hospital during 2017 - 2018. According to the findings, the median numerical per capita consumption of caspofungin increased before the implementation of the stewardship program compared to after the implementation of the plan (from 0.13 to 0.35), which was also observed in the case of linezolid (from 0.18 to 0.22). On the other hand, a reduction was denoted in the use of imipenem (from 2.78 to 2.38), amphotericin (from 0.48 to 0.14), teicoplanin (from 0.15 to 0.11), colistin (from 1.85 to 1.74), meropenem (from 1.09 to 1.00), voriconazole (from 0.40 to 0.20), and vancomycin (from 2.65 to 2.48). However, no significant correlation was observed between the median and numerical per capita deviation of antibiotic use before and after the stewardship program (P > 0.05) (Table 1).
| Antibiotics | Six Months Before | Six Months After | P-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Imipenem | 2.78 | 6.09 | 2.38 | 5.67 | 0.812 |
| Amphotericin B | 0.48 | 1.69 | 0.14 | 0.59 | 0.106 |
| Teicoplanin | 0.15 | 0.34 | 0.11 | 0.28 | 0.261 |
| Caspofungin | 0.13 | 0.26 | 0.35 | 5.31 | 0.135 |
| Colistin | 1.85 | 5.19 | 1.74 | 6.39 | 0.678 |
| Linezolid | 0.18 | 0.44 | 0.22 | 0.86 | 0.258 |
| Meropenem | 1.09 | 5.23 | 1.00 | 3.77 | 0.788 |
| Voriconazole | 0.40 | 4.50 | 0.20 | 0.78 | 0.459 |
| Vancomycin | 2.65 | 8.03 | 2.48 | 7.43 | 0.775 |
Median and IQR of Numerical per Capita Antibiotic Use Before and After Stewardship Program a
Before the implementation of the stewardship program, the median per capita consumption (Rials) of teicoplanin was 5.97 × 104, while it was 6.27 × 105 for caspofungin, and 1.90 × 105 for linezolid, which increased by 1.90 × 105, 1.28 × 106, and 2.16 × 105, respectively after the implementation of the plan. On the other hand, the median per capita consumption of imipenem decreased from 5.75 × 105 to 5.22 × 105, while it decreased from 1.21 × 106 to 3.94 × 104 for amphotericin, from 2.80 × 105 to 2.79 × 105 for colistin, from 1.18 × 106 to 6.02 × 105 for voriconazole, and from 1.93 × 105 to 1.83 × 105 for vancomycin. No significant correlation was observed between the median and IQR of Rials per antibiotic before and after the stewardship program (P > 0.05) (Table 2).
| Antibiotics | Six Months Before | Six Months After | P-Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Imipenem | 5.75 × 105 | 1.33 × 106 | 5.22 × 105 | 1.24 × 106 | 0.812 |
| Amphotericin B | 1.21 × 106 | 2.51 × 106 | 3.94 × 104 | 1.69 × 106 | 0.172 |
| Teicoplanin | 5.97 × 104 | 1.21 × 105 | 6.13 × 104 | 1.69 × 105 | 0.818 |
| Caspofungin | 6.27 × 105 | 1.63 × 105 | 1.28 × 106 | 1.80 × 107 | 0.098 |
| Colistin | 2.80 × 105 | 8.32 × 105 | 2.79 × 105 | 7.49 × 105 | 0.883 |
| Linezolid | 1.90 × 105 | 8.48 × 105 | 2.16 × 105 | 7.90 × 105 | 0.286 |
| Meropenem | 2.33 × 105 | 1.32 × 106 | 2.14 × 105 | 8.56 × 105 | 0.555 |
| Voriconazole | 1.18 × 106 | 1.35 × 107 | 6.02 × 105 | 2.34 × 106 | 0.494 |
| Vancomycin | 1.93 × 105 | 5.81 × 105 | 1.83 × 105 | 5.57 × 105 | 0.597 |
Median and IQR of Rials per Capita Antibiotic Consumption Before and After Stewardship Program (Million Rials) a
In the surgery ward, the median use of teicoplanin, caspofungin, linezolid, and meropenem increased after the implementation of the stewardship program, changing from 0.09 to 0.11, 0.22 to 5.73, 0.08 to 0.28, and 0.91 to 1.30, respectively. Meanwhile, the median of imipenem consumption decreased from 1.81 to 1.52, while it decreased from 9.09 to 3.15 for amphotericin, from 2.63 to 1.73 for colistin, from 9.67 to 0.26 for voriconazole, and from 1.49 to 1.22 for vancomycin. In the ICU, the median use of imipenem, caspofungin, and linezolid before and after the stewardship program increased by (3.12, 3.18), (0.12, 0.15), and (0.26, 0.77), respectively. However, a reduction was observed in the use of amphotericin (0.38, 0.09), teicoplanin (0.15, 0.08), colistin (2.28, 1.87), meropenem (3.15, 1.67), and vancomycin (3.02, 2.90).
In the internal medicine ward and before the implementation of the stewardship program, the consumption of caspofungin (0.10) and linezolid (0.10) increased by 0.21 and 0.14, respectively after the implementation of the plan. On the other hand, a reduction was observed in the median use of imipenem, amphotericin, teicoplanin, voriconazole, and vancomycin antibiotics from 4.74 to 3.97, 0.25 to 0.07, 0.18 to 0.12, 0.31 to 0.20, and 3.19 to 2.45, respectively. No significant correlation was denoted between the mean and numerical standard deviation of antibiotic use before and after the stewardship plan based on the hospital wards (P > 0.05) (Table 3).
| Antibiotics | Surgery Ward | ICU | Internal Medicine Ward | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Six Months Before | Six Months After | P-Value | Six Months Before | Six Months After | P-Value | Six Months Before | Six Months After | P-Value | |||||||
| med | IQR | med | IQR | med | IQR | med | IQR | med | IQR | med | IQR | ||||
| Imipenem | 1.81 | 4.72 | 1.52 | 3.63 | 0.614 | 3.12 | 6.10 | 3.18 | 4.56 | 0.667 | 4.74 | 6.80 | 3.97 | 5.99 | 0.691 |
| Amphotericin B | 9.09 | 0.01 | 3.15 | 11.28 | 0.643 | 0.38 | 0.73 | 0.09 | 0.26 | 0.114 | 0.25 | 1.10 | 0.07 | 0.30 | 0.06 |
| Teicoplanin | 0.09 | 0.33 | 0.11 | 0.19 | 0.713 | 0.15 | 0.32 | 0.08 | 0.30 | 0.339 | 0.18 | 0.35 | 0.12 | 0.35 | 0.309 |
| Caspofungin | 0.22 | 5.49 | 5.73 | 6.75 | 0.224 | 0.12 | 0.21 | 0.15 | 0.01 | 0.905 | 0.10 | 0.21 | 0.21 | 0.32 | 0.181 |
| Colistin | 2.63 | 6.24 | 1.73 | 18.09 | 1.00 | 2.28 | 4.61 | 1.87 | 3.37 | 0.701 | 0.37 | 3.31 | - | - | - |
| Linezolid | 0.08 | 0.66 | 0.28 | 1.34 | 0.520 | 0.26 | 0.54 | 0.77 | 1.38 | 0.45 | 0.10 | 0.38 | 0.14 | 0.16 | 0.064 |
| Meropenem | 0.91 | 4.99 | 1.30 | 4.62 | 0.499 | 3.15 | 8.07 | 1.61 | 7.37 | 0.785 | 0.98 | 1.91 | 0.51 | 1.15 | 0.087 |
| Voriconazole | 9.67 | 0.01 | 0.26 | 3.67 | 0.096 | - | - | 0.46 | 1.70 | - | 0.31 | 0.39 | 0.20 | 0.01 | 0.630 |
| Vancomycin | 1.49 | 8.70 | 1.22 | 8.65 | 0.738 | 3.02 | 13.26 | 2.90 | 14.24 | 0.553 | 3.19 | 3.99 | 2.45 | 2.51 | 0.432 |
Median and IQR of Antibiotic Consumption per Capita by Hospital Ward Before and After Stewardship Program a
5. Discussion
The discovery of antibiotics led to a significant reduction in the incidence of infectious diseases associated with high mortality. However, factors such as the improper administration, misuse, and overuse of antibiotics have caused antibiotic resistance, which is currently a global health threat. Given the importance of this issue, the present study aimed to compare the use of antibiotics before and after implementing the stewardship program in Imam Reza Educational and Medical Center in Kermanshah, Iran. Considering the structure of the stewardship program, restrictive interventions without increasing mortality, increased length of hospital stay, or increased drug-related side-effects could be responsible (8).
Our findings indicated that the median per capita numerical consumption of caspofungin and linezolid before the implementation of the stewardship program increased compared to after the implementation of the plan, while a reduction was observed in the use of imipenem, amphotericin, teicoplanin, colistin, meropenem, voriconazole, and vancomycin. Nevertheless, no significant correlation was observed between the median and per capita numerical deviation of antibiotic use before and after the stewardship program.
A study conducted by Cai et al. in a hospital in Europe indicated that vancomycin and meropenem use decreased during the stewardship program (9). Furthermore, a systematic review performed by Van Dijck in low- and middle-income countries showed that antibiotic use decreased following the initiation of this program (5). Another study regarding the measurement of antibiotic use after the stewardship program indicated that inappropriate antibiotic use decreased after the plan was implemented (10). In addition, the study conducted by Kisuule et al. on 247 cases before the stewardship program and 129 cases after the program showed that the inappropriate use of antibiotics increased from 57% before the plan to 26% after the plan (11).
A study conducted at the Children's Hospital of Philadelphia demonstrated that the intervention of prescribing broad-spectrum antibiotics to children during primary care was almost halved, and the use of unnecessary antibiotics for children with pneumonia reduced by up to 75% after the intervention (12). Similarly, a study conducted in Japan during 2016 - 2013 showed that the national stewardship program reduced antibiotic use by 50% in hospitalized patients (13). In Brazil, the implementation of the stewardship program resulted in 11.3% reduction in antibiotic use and 53% reduction in treatment costs (14). The stewardship program was also reported to be effective in Israel, and a study indicated that during 2012 - 2017, antimicrobial use declined significantly (15). According to the findings of Malani et al., caspofungin use decreased by 50% after the stewardship program (16). Moreover, the results obtained by Magedanz et al. in Brazil indicated that the use of antibiotics decreased after implementing the stewardship program (17).
The stewardship program shows that education has a significant impact on reducing the need for unnecessary antibiotics. The insufficient knowledge of physicians about the proper use of antibiotics and the pressure of the patients who believe that antibiotics could rapidly reduce the symptoms of their disease are among the most important influential factors in antibiotic overuse. Therefore, existing antibiotics should be used more responsibly and managed carefully so that their lifespan would increase. The main goal of the stewardship program is to reduce the misuse of antimicrobial drugs, improve patient outcomes, reduce the side-effects of antibiotic treatment, decrease the incidence and spread of infections, and diminish the overall costs of treatment. Moreover, the stewardship program converts intravenous antibiotics into oral antibiotics, restricts the range of antibiotics, and limits the length of treatment as recommended by national and international guidelines.
According to the findings of the current research, the median per capita consumption of Rials increased in the case of teicoplanin, caspofungin, and linezolid, while a reduction was observed in the use of amphotericin, colistin, voriconazole, and vancomycin after the implementation of the stewardship program. However, no significant correlation was observed between the median and IQR of Rial per antibiotic before and after the stewardship program.
Another study in this regard was conducted by Hajiabdolbaghi et al. in Tehran (Iran) on patients with infectious diseases, and the obtained results indicated that the costs of antibiotics decreased after the stewardship program (6). Another study showed that in 2012 and after the introduction of the stewardship plan, the costs of antibiotics decreased compared to 2010 (before the stewardship plan) (18). In addition, a two-stage study conducted by Niwa et al. in Japanese hospitals indicated that the annual costs of antibiotic injection decreased from $2.02 million to $0.2 million in the first period and $1.86 million in the second period (10). It is hoped that further investigations evaluate antibiotics use on a larger scale and determine the potential problems associated with antibiotic restriction, as well as the possible solutions to these problems.
5.1. Conclusions
According to the results, the frequency of using antibiotics such as vancomycin was higher than other antibiotics, while teicoplanin was the least commonly used antibiotic agent in the selected hospital wards. Only a slight reduction was observed in the prescription of the studied antibiotics after the implementation of the stewardship program, indicating that adherence to the program has been improper, and the prescription of antibiotics has not changed significantly. Moreover, training in this regard has been dissatisfactory and inadequate, and executives such as physicians and residents are not sufficiently familiar with the stewardship program or have failed to practically comply with its principles. Infectious disease specialists may have also lacked the necessary skills or cooperation to stop the administration of unnecessary antibiotics.
Observations show that although other physicians than infectious disease specialists could only prescribe certain antibiotic within the first 24 hours after the patient’s admission, infectious disease specialists have allowed the continuation of antibiotics without considering the standards or evaluating the conditions of patients. The time span of the current research was not sufficient for a proper conclusion. Therefore, the quality of the implementation of the stewardship program should be evaluated in further studies. Hospital infection control committees should also consider more training sessions and meetings for their healthcare team and physicians.