1. Background
The essential element iron plays a vital role in cell proliferation, metabolism, and growth (growth-limiting factor) in almost all organisms (1). Iron is essential for oxygen-carrying, DNA synthesis, enzyme and redox catalysis, energy production, and cellular respiration (2). Iron metabolism dysregulation can cause a wide range of diseases, and understanding its role is vital in therapeutics search (3).
Since humans have no active mechanism for excreting iron, iron absorption by the proximal small intestine is strictly controlled. The epithelial cells of the upper jejunum and upper and mid villus in the duodenum are the first mediators of intestinal iron absorption (4). Dietary iron is imported across the brush-border membrane, stored in ferritin (an intracellular stage), exported across the basolateral membrane (4, 5). Studies have shown that iron absorption increases in the presence of ascorbic acid as a reducing agent for changing ferric (III) to ferrous (II) and glucose as an energy source for transporting iron throughout the cells (6, 7).
Iron uptake has been studied extensively over the past few decades, but its possible interference with some trace elements has not received the attention it deserves. Lanthanum is a chemical element composed of stable La-139 (99.911%) and (ii) radioactive La-138 (0.089%) isotopes (8). Various food items and tap water contain trace amounts of this rare-earth element. This cation of trivalent hard acid exhibits a high affinity for phosphate and pH independence (9). The use of lanthanum in medicine has been proven in several studies, which can be used as a safe inorganic phosphate binder in patients on dialysis (10). Ascorbic acid enhances iron absorption the most among organic acids because of its chelating and reducing properties (11). Glucose, in particular, affects iron bioavailability and can chelate mineral iron, forming low molecular weight soluble complexes, which increases iron absorption (12).
2. Objectives
The study of iron metabolism, especially its absorption process, is essential due to its vital role in the human body. The effect of trace elements on iron absorption is an area of great interest, but the effect of lanthanum on iron absorption has not yet been studied. Hence, this study aimed to evaluate the intestinal uptake of lanthanum and its competition with iron uptake.
3. Methods
The everted gut sac (EGS) method was used to evaluate the intestinal uptake of iron and lanthanum. In these studies, the method is useful for examining the in-vitro absorption mechanisms of drugs, the roles of transporters in drug absorption, intestinal metabolism of drugs, and the role of intestinal enzymes in the transport of drugs through the intestine (13). The materials were provided by Sigma Chemical Company U.K. Male Wistar rats were obtained from Falavarjan Islamic Azad University (Isfahan, Iran) and were kept in standard condition until they reached 200 - 250 g. In the EGS model, rats were fasted one day before the experiment and anesthetized with ether. Then, the duodenum, ileum, or jejunum of their intestine was rapidly removed and divided into 5 - 6 cm segments. Each segment was washed with a physiological solution (pH 6.5, 4°C). The glassware used in the experiment was soaked overnight in nitric acid 10%. Then, the washed intestine was gently everted over a glass rod. One end of the everted intestine was clamped and tied with a braided silk suture and filled with 2 mL krebs-ringer bicarbonate (KRB) solution at 37°C. Then, the other end of the segment was tied with a thread. EGS segments were ready to be examined for the effect of various factors (13). A study of iron absorption in the form of ferric was conducted to investigate iron absorption.
The EGS segments were first placed in separate test tubes containing 5 mL of krebs-ringer bicarbonate buffer (pH 7.4) to determine the optimal concentration for the intestinal absorption of iron and lanthanum by EGS. Then, a mixture of oxygen-carbon dioxide (95: 5) and various concentrations of iron and lanthanum were complexed with citrate (0 - 200 mg/L). Then, iron (III) chloride, and lanthanum (III) chloride were prepared in bi-distilled water and mixed with equal volumes of citric acid 1:20. The contents of the EGS segments were removed after 60 min of incubation at 37°C. The concentrations of iron and lanthanum were measured using uv2600 Spectrophotometry (Shimadzu, Japan). By using inductively coupled plasma (ICP) technology, 100 μL of EGS segments' content were diluted with 100 μL of nitric acid 0.2% and the lanthanum concentration was determined at 240 nm wavelength (13).
A total of 2 mL of Earls buffer (pH 7.4) was added to the EGS segments, which were inserted into the test tube containing 5 mL of the Earls's buffer and different concentrations of iron and lanthanum. Besides these substances, glucose (5 mM) and ascorbic acid (2.8 mM) were tested for their effects on lanthanum and iron uptake at different concentrations (0 - 200 mg/L). The concentration of iron and lanthanum in the EGS segments was investigated after incubation at 37°C in line with the previous methods (14).
The same steps (mentioned above) were followed to investigate the interfering effect of lanthanum on intestinal iron absorption. 2 mL of Earls buffer (pH 7.4) was added into the EGS segments. These segments were inserted into the test tube containing 5 mL of Earls's buffer. In this two-stage test, the iron intestinal absorption process was investigated (0 - 200 mg/L) in the presence (100 mg/L) and absence of lanthanum for its different concentrations. In the next stage, the iron intestinal absorption process for different concentrations of lanthanum (0 - 200 mg/L) was investigated in the presence (100 mg/L) and absence of iron. Iron and lanthanum concentrations were measured after 60 minutes of incubation (37°C) (14).
The elements’ uptake was examined at different time intervals (15 minutes) to investigate the effect of time on intestinal uptake. The EGS segments were removed from the medium, and the iron and lanthanum concentrations were measured at different time intervals, which was 100 mg/L in each experiment (14).
The data were expressed in mean ± standard error and analyzed by two-way ANOVA using GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA, USA). A statistically significant difference between groups is indicated by P < 0.05.
4. Results
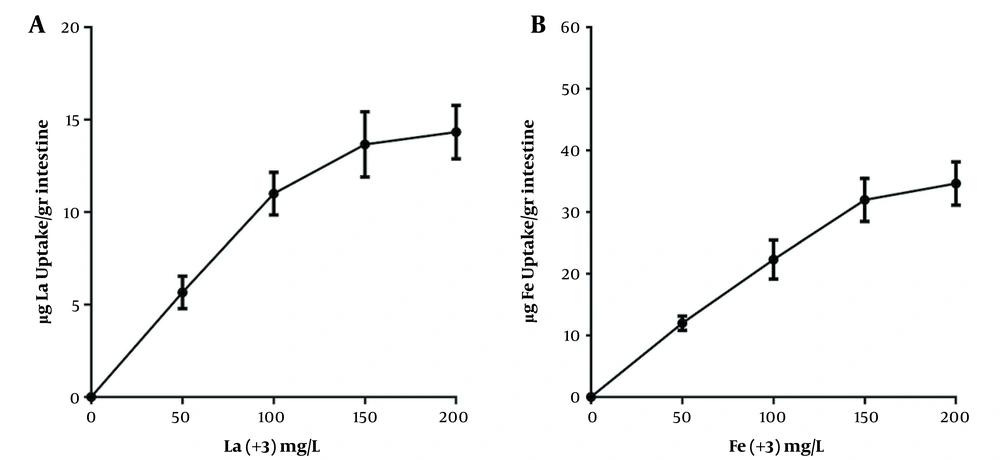
Since citrate is an iron chelator, iron was complexed with citrate to examine iron absorption. Therefore, each piece was filled with 2 mL of Earls's buffer and the ends of the intestine segments were closed with surgical sutures after preparing the EGS segments. Then, the EGS segments were placed in separate mediums containing iron citrate with different concentrations. Mediums were then exposed to oxygen at 37°C for 30 min for iron absorption, and the amount of iron absorbed from the incubation medium was measured. Similar experiments were performed with different concentrations of lanthanum. Figure 1 demonstrates that intestinal absorption of these elements increases as the concentration of iron and lanthanum in the medium increases. The results showed that the maximum intestinal absorption is 200 mg/L for iron and lanthanum.
Determining the optimal concentration of intestinal absorption of lanthanum by the EGS. A, iron intestinal uptake; B, lanthanum intestinal uptake. Each point is the average of three independent experiments in mean ± standard deviation. Lanthanum complexed with citrate (1:20) at 37°C for 60 minutes, and the EGS segments average weight was 1 gr.
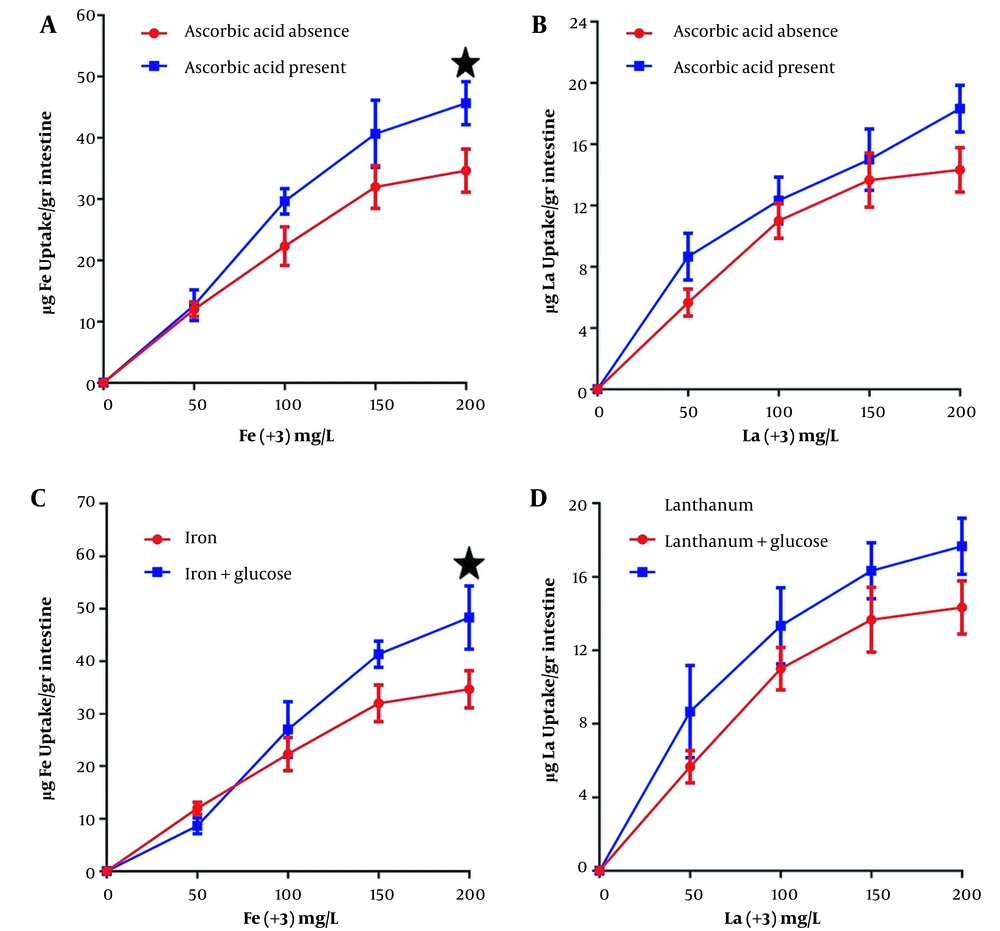
Figure 2 shows the effects of ascorbic acid and glucose on iron and lanthanum uptake, which increases intestinal absorption of iron and lanthanum in the incubation medium at 200 mg/L concentration. The intestinal iron absorption at 200 mg/L increases by 29.0% in the presence of ascorbic acid compared to its absence, and the lanthanum uptake at the highest concentration in the presence of ascorbic acid also increase by 8.3%. Both iron and lanthanum absorption increased in the presence of glucose by 2.9% and 25.7%, respectively.
The comparison of iron and lanthanum intestinal uptake in the presence and absence of ascorbic acid and glucose. The ascorbic acid and glucose concentrations added to the EGS external medium are 2.8 mM and 5 mM, respectively. Each point is the average of three independent experiments in mean ± standard deviation. Lanthanum complexed with citrate (1: 20) at 37°C for 60 minutes, and the EGS segments average weight was 1 g. Each point represents the group mean (mean ± standard error of the mean) (*: P < 0.05, **: P < 0.01).
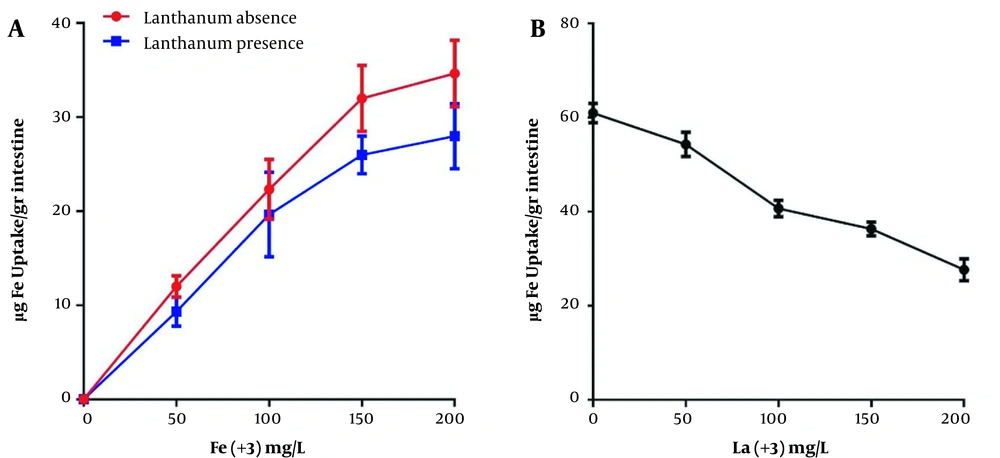
Figure 3 illustrates the effect of constant and variable concentrations of lanthanum on intestinal iron uptake. According to the results, lanthanum interferes with iron intestinal uptake andiron uptake in the EGS segments. The intestinal uptake of iron reduces by 19.7% in the maximum concentration of iron in the presence of constant concertation of lanthanum. Figure 3B presents that iron absorption decreases gradually as lanthanum concentration increases (54.6% reduction in the highest lanthanum concentration compared to the absence of lanthanum).
A, The presence and absence of lanthanum on intestinal uptake of iron; and B, the different concentration effect of lanthanum on intestinal uptake of iron. The constant concentration of iron used in the (B) chart is 100 mg/L. Each point is the average of three independent experiments in mean ± standard deviation. Lanthanum complexed with citrate (1: 20) at 37°C for 60 minutes, and the EGS segments average weight was 1 g. Each point presents the group mean (mean ± standard error of the mean (*: P < 0.05, **: P < 0.01).
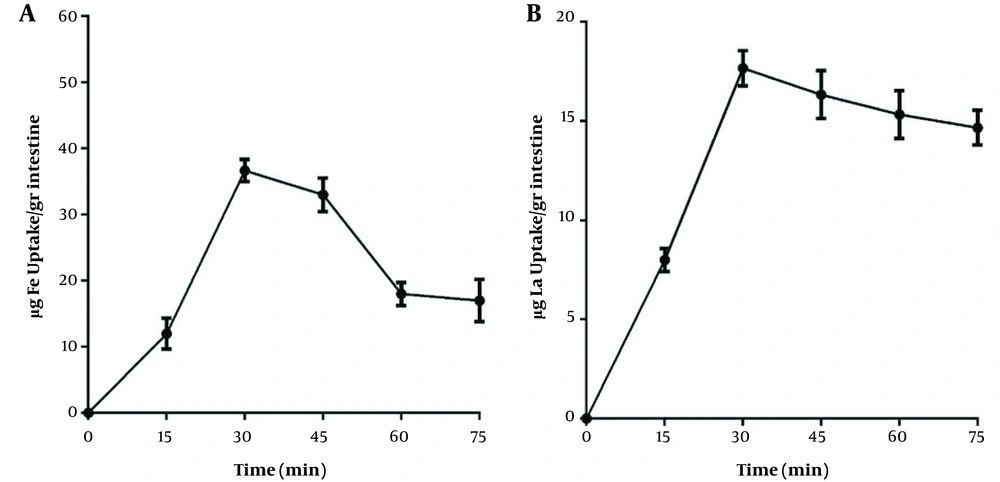
The effect of time intervals on iron and lanthanum intestinal absorption are shown in Figure 4. The results reveal that the highest uptake occurs in 30 minutes in which, the average uptakes for iron and lanthanum are 36.6 and 17.6 μg, respectively.
The effect of time on the intestinal absorption of A, iron; and B, lanthanum. The EGS segments were removed from medium, and the concentrations of iron and lanthanum were measured at different intervals. The iron and lanthanum concentration was 100 mg/L in each experiment. Each point is the average of three independent experiments in mean ± standard deviation. The lanthanum complexed with citrate (1: 20) at 37°C, for 60 minutes, and the EGS segments average weight was 1 g.
5. Discussion
The intestinal uptake of lanthanum and iron by the EGS and their interaction were initially investigated. First, the optimal intestinal absorption of lanthanum and iron concentrations was investigated in the incubation medium. The iron and lanthanum uptake was investigated at different concentrations by the EGS The results showed the concentration of lanthanum and iron in the incubation medium increases by raising its absorption.
Iron deficiency is usually related to low Fe intake, poor iron absorption, blood loss, diseases, gastrointestinal parasites, or increased physiological demands as in pregnancy (15). The intestinal absorption of trace elements such as copper, zinc, and nickel. is carried out through an active transfer mechanism (16, 17). Previous studies have shown that iron transfer from intestinal mucosal cells is energy-dependent, which is absorbed through an active transfer mechanism, and the energy required for its absorption is provided by cellular metabolism (18). The present study examined iron and lanthanum uptake mechanisms to answer examine the energy dependence of their transport through intestinal mucosal cells and their absorption by the EGS in the presence of glucose? The results showed that, iron and lanthanum absorption and the possibility of the active transfer of iron and lanthanum increases in the presence of glucose in the incubation medium. This result is consistent with that of previous studies showing that glucose increases iron absorption in the gut (12).
Although iron can be absorbed in ferrous (II) and ferric (III) forms in intestinal mucosal cells, the absorption of ferrous form is high, and ascorbic acid was used to reduce iron (III) (7, 19). Ascorbic acid significantly increases iron absorption and the intestinal absorption of lanthanum, but it is not significant. The results revealed that reducing these elements causes an increase in intestinal absorption.
Studies have shown that, various proteins are involved in the transport and storage of iron in these cells in intestinal mucosal cells. These proteins include transferrin and ferritin, which play a controlling role in the absorption of iron in mucosal cells (20, 21). The transferrin in intestinal mucosal cells causes this protein may also play a role in the intestinal absorption of lanthanum. This study results also found that iron absorption reduces slightly in the presence of lanthanum.
As shown in Figure 3, the lanthanum interfering effect was studied by EGS in the intestinal absorption of iron. At high concentrations of lanthanum, iron absorption gradually decreases, confirming lanthanum's interfering effect on iron absorption. In addition to the role transferrin likely plays in the intestinal absorption of lanthanum, which is well known, the transferrin molecule has also been linked to intestinal iron absorption (22). Therefore, lanthanum is probably absorbed by intestinal mucosal cells with the same mechanism as iron. Due to transferrin's role in intestinal iron absorption, these two elements can compete at the absorption site and interfere with each other's metabolism. In previous reports, the interference effect of trivalent cations has been proven in the binding of iron to apotransferrin, which is consistent with our results (23).
The effect of incubation time was also investigated on the adsorption of these two elements. Using the EGS to measure intestinal absorption of iron and lanthanum, it was found that the maximum absorption occurred around 30 minutes, and then the absorption decreased. The decreased absorption may be due to the loss of intestinal mucosal cell life. However, all physiological conditions have been considered in carrying out this project. Previous studies have also shown that iron uptake by the intestine occurs 5 - 30 minutes after incubation, which agrees with the findings of this study (24, 25).
5.1. Conclusions
The results of this study revealed that lanthanum could affect the first step of intestinal iron absorption. However, more investigation is needed at the molecular and intracellular levels to better understand lanthanum interference with iron uptake and its metabolism.