1. Background
Several studies have been recently published directly and indirectly on the effects of herbal medicines extracted from natural sources on lncRNA (1-3). In addition to regulating gene expression, lncRNAs participate in cell cycle regulation, apoptosis, survival, proliferation, differentiation, and migration (4-6). lncRNAs play an important role in metastasis and invasion of cancer cells. In recent years, colorectal cancer (CRC) has become increasingly prevalent and many people die from this disease each year. The lack of early detection and unknown molecular mechanisms are two factors leading to high mortality (7-10). The role of lncRNAs in developing and progressing colon cancer has recently been discovered (11-17). In a study, Zhang et al. found that hnRNP-K targeting increased nuclear β-catenin accumulation and activated the β-catenin-Wnt pathway in CRC patients, which increased cell proliferation, migration, and invasion (12). Another study found a significant increase in SBDSP1 expression in CRC tissues comparing CRC tissues with normal tissues. SBDSP1 increases the function of Akt and ERK1,2 pathways, and SBDSP1 silencing reduces the viability and the expression of cyclin D1, and thus stops the cell cycle in G0/G1 phase in cancer cells. Another study found that increased LINC00460 expression increased colon cancer cell proliferation, cell division, and tumor growth in vivo. Furthermore, decreased E-cadherin expression and increased vimentin and N-cadherin expression induced EMT (13). Wang et al. showed that the ROR1-AS1 level increased significantly in colon cancer tissues and cell lines at stages 4 and 3 in comparison to stages 1 and 2, enhanced cell proliferation, cell cycle progression, and apoptosis inhibition, and suppressed DUSP5/CDKN1A expression (14).
Medicinal plants produce active ingredients, such as curcumin, paclitaxel, silibinin, gambogic acid, genistein, which inhibit cancer cell survival, migration, invasion, and metastasis by altering the function of long noncoding RNAs and increase the sensitivity of cancer cells to radiotherapy and chemotherapy (1-3). Silymarin is a flavonoid compound derived from the Silybum marianum plant, which contains the three main isomers of silibinin, silydianin, and silychristin with the most significant effects of the plant on this category of substances. In vitro and in vivo studies have revealed that silymarin and silibinin have anti-cancer properties (18).
There is a CASC11 gene on chromosome 8q24, 2.1 kb upstream of c-Myc, and a SBDSP1 gene on chromosome 11q37. The expression level of these lncRNAs increases in patients with CRC, depends on tumor size, lymph metastasis, and serous invasion (TNM staging) (19), and their inhibition prevents the proliferation and metastasis of colon cancer cells (19). There are few studies on the effects of silymarin on CASC11 and SBDSP1. This study evaluated the effect of silymarin on gene expression and induction of cell apoptosis by CASC11 and SBDSP1 lncRNAs in the HCT-116 colon cancer cell line.
2. Methods
In this study, fetal bovine serum (FBS), DMEM medium, penicillin, streptomycin, MTT assay dye, trypan blue, and other high purity reagents were obtained by Sigma, and Barij Essence Company prepared Silymarin in powder form.
2.1. Cell Culture
The cells used in this study came from the Pasteur Institute (Tehran, Iran) Cell Bank’s HCT-116 colon cancer cell line, which are related to the human colon. These cells were cultured in a DMEM medium containing 100 mL L-glutamine, 10% FBS, 100 units/mL penicillin, and 100 g/L streptomycins, located in incubator containing 5% CO2 at 37°C.
2.2. Incubation of Cells with Different Concentrations of Silymarin
The cells were treated with Silymarin, purchased from Barij Essence Company. Simultaneously, purchased silymarin powder was prepared in sterile DMSO solution at 6.25, 12.5, 25, 50, 100, 250, and 500 μg/mL.
2.3. Evaluation of Silymarin Cytotoxicity by MTT Assay
MTT colorimetric method was used to evaluate the effect of Silymarin on the growth and proliferation of normal and cancer cells based on the ability of live cells to convert tetrazolium salts to insoluble formazan.
HCT-116 cells were cultured in a 96-well plate with 103 × 6 cells in each well in 200 μL of DMEM medium and three wells serving as the untreated control group.
Cells were treated at 6.25, 12.5, 25, 50, 100, 250, and 500 μg/mL for 24, 48, and 72 hours after 24 hours of incubation at 37°C. The supernatant was removed at the end of the treatment period, and 50 microliters of MTT solution (5 mg/mL) were poured into each well. Then, 100 μL of DMSO solvent were added to each well. ELISA measured the light absorption of the samples at 570 nm after 15 minutes of incubation at room temperature and complete dissolution of the crystals. Experiments were performed in triplicate to increase work productivity and comparative study.
2.4. Evaluating the Induction of Cellular Apoptosis Using Annexin/PI Kit
Studying phosphatidylserine molecules on the outer surface of the membrane is one of the ways to evaluate apoptosis during the apoptotic process. The presence of phosphatidylserine (PS) on the cell surface, which is usually present in the plasma membrane, is one of the early signs of apoptosis. Phosphatidylserine moves from the cytoplasmic surface of the plasma membrane to the extracellular surface during apoptosis. Ca2+ ions strongly bind annexin (An)-V, which can therefore be used as an apoptosis marker in the presence of Ca2+ ions.
The percentage of apoptosis and necrosis was determined using a bioscience diagnostic kit. The cells were incubated in a 6-well plate in the DMEM medium containing 10% bovine fetal serum and 5% CO2 at 37°C for 24 hours. Then, concentrations lower than the IC50 determined were added to the wells three times by the MTT method, and incubation lasted for 48 hours. Phosphate-buffered saline PBS was used to wash the cells twice after treatment. PI and An-V were added to the cell suspension in the binding buffer. The cells were incubated for 37 minutes at 37°C in the dark and analyzed by flow cytometry (BD Biosciences FACS CalibureTM).
2.5. RNA Extraction and qRT-PCR
The YT9080 kit was used to extract total RNA, and the YT4500 kit was utilized to synthesize cDNA according to the manufacturer’s instructions. The quantitative real-time PCR was performed in triplicate using QPCR Master MIX SYBR Green I (amplicon). A normalized GAPDH gene was used as an endogenous control gene, and results were analyzed using the ∆∆CT-2 (Livak) method. The sequence of primers used in qRT-PCR was used according to Table 1 (11, 20). All real-time reactions were performed using an ABI-step one device.
| Gene | Primer Sequence |
|---|---|
| CASC11 | Forward: 5’-GGACACCAACTATTGCTTCA-3’ |
| Reverse: 5’-TCCAGGCTCCAAATGTAGG-3’ | |
| SBDSP1 | Forward: 5’-GCTTCTGGGTCTTTGAACAGCC-3’ |
| Reverse: 5’-TTGGCAACCTGACCTTAGGAAAC-3’ | |
| GAPDH | Forward: 5’-AGAGGCAGGGATGATGTTCTG-3’ |
| Reverse: 5’-GACTCATGACCACAGTCCATGC-3’ |
2.6. Statistical Analysis
GraphPad Prism 8.3.0 Software, one-way ANOVA and Tukey’s multiple comparison tests were used for statistical analysis and graphs. The mean difference of the data was considered significant (P ≤ 0.05).
3. Results
3.1. Evaluation of Cell Cytotoxicity by MTT Assay
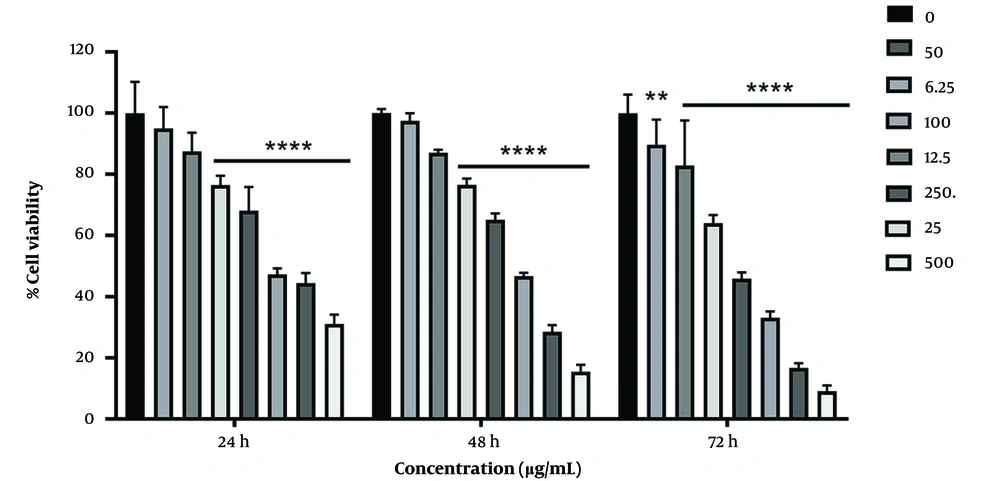
The MTT test results showed that silymarin treatment increases in cell death in HCT-116 colon cancer cells, depending on the concentration and time.
In Figure 1, the survival rate of HCT-116 cancer cells was lower at all concentrations and times when compared to the control group. The survival percentage decreased significantly in the concentration range of 0 - 500 g/mL at 24 and 48 hours (P 0.001), as well as at 6.25, 12.5, 25, 50, 100, 250, and 500 g/mL at 72 hours (P 0.001, P 0.01). During 24 hours of treatment, the highest survival rate was observed at a concentration of 6.25 g/mL (97.49%), while the lowest survival rate was observed at 500 g/mL (9.106%) during 72 hours of silymarin treatment. The level of IC50 in the HCT-116 cancer cell line in MTT at 24, 48, and 72 hours was observed 141.8, 91.73, and 47.52 μg/ml, respectively.
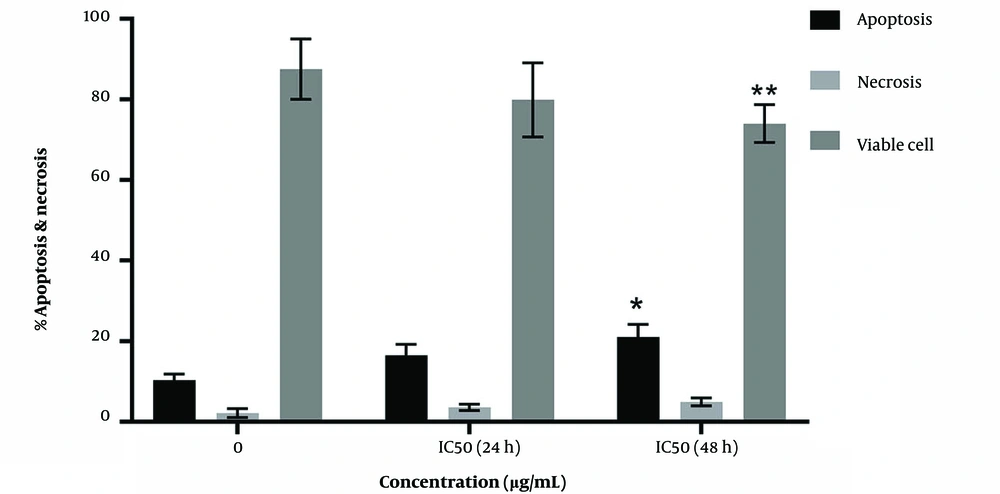
3.2. Results of Apoptosis with Annexin/PI Kit
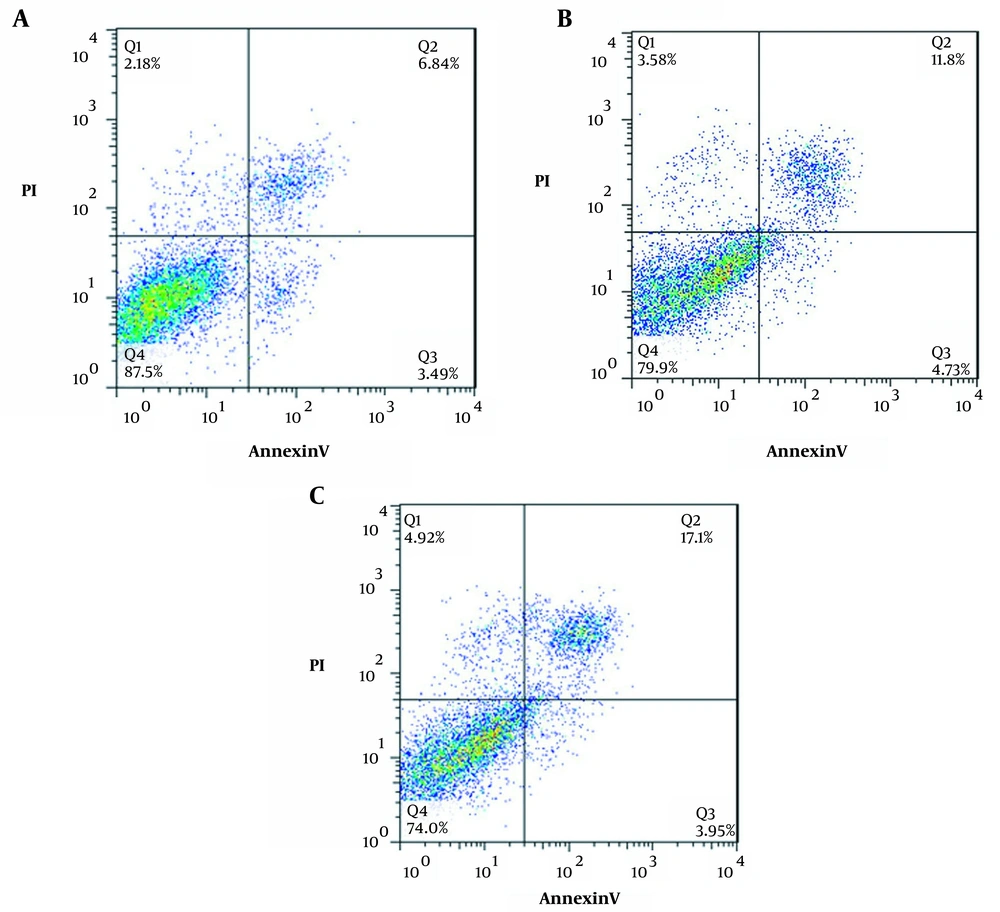
Figures 2 and 3 indicate a significant increase in apoptosis percentages of 16.53 ± 1.5 (P = 0.24) and 21.05 ± 1.79 (P = 0.05) in treatment with IC50 concentrations for 24 and 48 hours, respectively, compared to the control (10.33 ± 0.86). In addition, the percentage of necrosis in silymarin treatment increased by 58.3 ± 0.46 (P = 0.924) and 4.920 ± 0.572 (P = 0.743) for 24 and 48 hours, respectively, compared to the control, (2.180 ± 0.63). In addition, a significant decrease was observed in survival at 79.9 ± 5.5.312 (P = 0.128) and 74.7 ± 2.714 (P < 0.01) compared to the control (87.5 ± 4.33).
The percentage of cells in different phases of annexin (An)-V/PI test in HCT-116 cell line for 24 and 48 hours. A, Control; B, treatment at IC50 concentration for 24 hours; C, treatment at IC50 concentration for 48 hours; Q1: marker of An-/pI+ necrosis cells, Q2: marker of An+/pI+ delayed apoptotic cells, Q3: primary apoptotic marker of An+/pI-, Q4: An-/pI- live cells
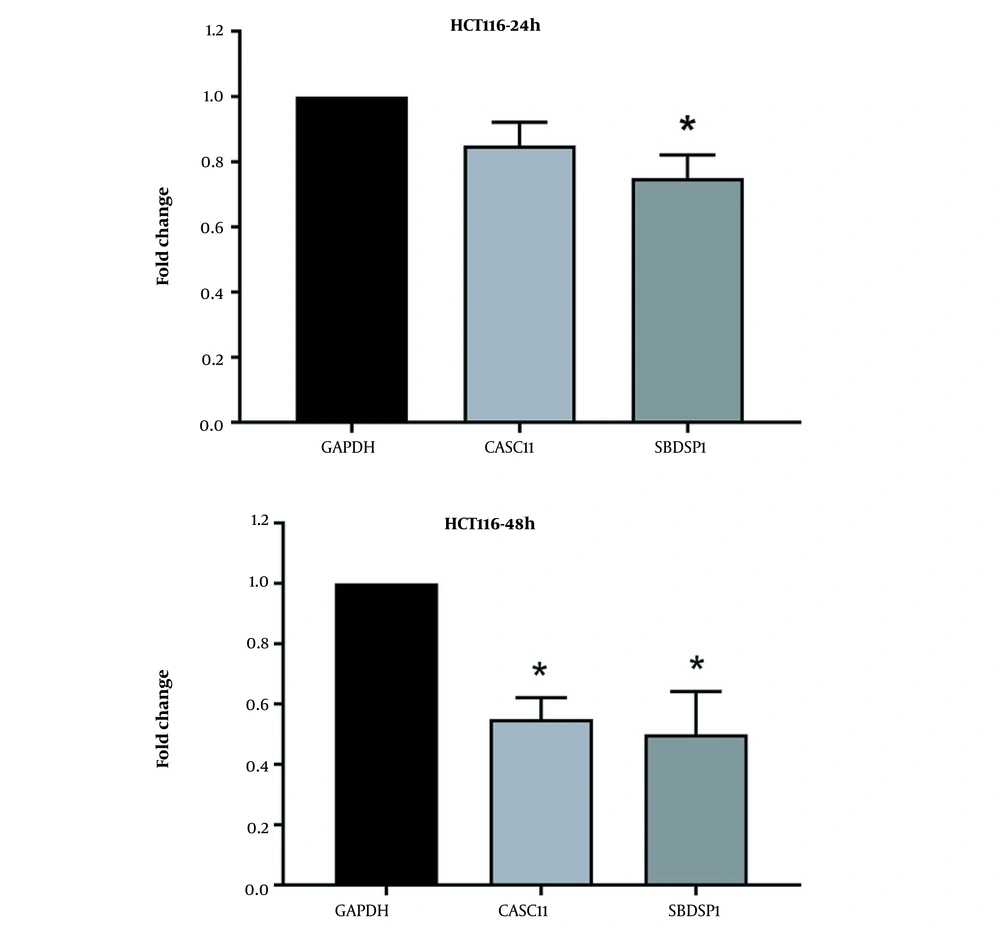
3.3. Results of Evaluating Gene Expression
As shown in Figure 4 the expression of the SBDSP1 gene decreased by 1.3 times compared to the control group in 24 hours. In addition, the expression of SBDSP1 and CASC11 genes decreased significantly by 2 and 1.8 times compared to the control group in 48 hours, respectively (P ≤ 0.05).
4. Discussion
In this study, silymarin-treated cells significantly decreased the survival percentage and increased the percentage of apoptosis. The lncRNA expression of SBDS1 and CASC11 RNAs significantly decreased when compared to the control group. A number of cancers have been associated with an increase in the expression of CASC11 and SBDSP1 lncRNAs (11, 13). Oncogenic lncRNAs inhibit tumor inhibitors or induce antiapoptotic regulators. Su et al. found an increase in CASC11 and CDK1 expression in gastric cancer cells and tissues and a link between increased CASC11 expression and tumor size, TNM stage, cellular apoptosis inhibition, and increased cell cycle progression (21). Another study reported an increased CASC11 expression in ovarian cancer tissues and cells and its relationship with a decrease in mir-182, inhibition of cellular apoptosis, and increased cell proliferation (22). Zhang et al. reported an increase in CASC11 in CRC patients, which activated the β-catenin/Wnt pathway by raising the accumulation of nuclear β-catenin and enhancing cell proliferation, migration, and invasion (12). Zhou et al. concluded that the relative expression of SBDSP1 is significantly increased in CRC tissues compared to normal ones (15). The increase in relative expression is associated with invasive symptoms of cancer cells. Silencing the SBDSP1 gene reduced viability, increased cessation of cancer cells in G0/G1 phase, and inhibited the expression of D1 genes in CRC samples with metastatic nodes. According to in vivo studies, the decrease in SBDSP1 expression reduces and delays the growth of xenograft tumors in colon cancer (15).
Herbal drugs such as curcumin, silibinin, genistein, resveratrol, berberine, paclitaxel, and others have been shown to interact with lncRNAs and their functional regulation (1-3).
These plant compounds directly or indirectly affect the expression of lncRNAs by interacting with miRNAs, protein kinases, enzymes, and transcription factors, modulating the expression of lncRNAs leads to inhibition of survival, proliferation, migration, invasion, metastasis, and transmitting from the epithelium to mesenchyme of cancer cells (1-3).
On the other hand, Zhang et al. reported the effect of CASC11 on increasing the Wnt/B catenin pathway activity by hnRNP-K targeting (12). Therefore, the results of previous studies are consistent with the present study based on the effect of silymarin in reducing the expression of CASC11 genes, decreasing cell proliferation, and increasing apoptosis in the HCT-116 cell line (12).
In the current study, silymarin inhibited colon cancer cell proliferation by affecting the expression of the SBDSP1 and CASC11 genes. The present study has limitations regarding the effect of silymarin on tumor suppressor expression of genes, caspases, and measuring free radicals and cell cycle in colon cancer cells. The results of this study can be used to determine the effects of silymarin on the expression of lncRNA genes associated with colon cancer and prevent their expression.
The results of previous studies and the current findings suggest that silymarin inhibits CASC11 and SBDSP1 in HCT-116 cells, which should be explored further. Herbal medicine interactions with lncRNA provide valuable information for identifying treatment strategies for diseases associated with a sudden increase in lncRNAs, and they need to be investigated further.
4.1. Conclusions
According to the results, SBDSP1 and CASC11 lncRNAs are overexpressed in colon cancer cell lines and tissues. Silymarin is a flavonoid extracted from the plant S. marianum with anti-cancer properties by regulating messaging pathways and interacting with lncRNAs in various cancers (1-3). This study found that silymarin treatment reduced cell proliferation, increased apoptosis, and decreased expression of oncogene long noncoding RNAs SBDSP1 and CASC11. Consequently, silymarin could control the function of lncRNAs.