1. Background
Menopause increases the risk of cardiovascular disease (CVD) (1). Cardiovascular disease results in high mortality and morbidity following menopause (2). Recent studies have shown the effectiveness of isoflavones (IFs) in the prevention of various chronic illnesses (3, 4). Soy IF supplementation is an alternative method in controlling lipid metabolism in menopausal women to prevent CVD without deleterious side effects (5). Soy IF as a phytoestrogen similar to 17b-estradiol can bind to estrogen receptors b and a and prevent CVD through a reduction in oxidation of LDL-C and an increase in nitric oxide (NO) (6).
Studies have reported that soy IFs are effective for postmenopausal women who have type 2 diabetes (7). Moreover, the biological effects (8, 9) and effectiveness of IFs could prevent many chronic diseases, such as CVD (10, 11).
The benefits of exercise training have been noted in most organ systems, such as the cardiovascular, respiratory, neuroendocrine, and musculoskeletal systems (12). High-intensity interval training (HIIT) can take a form as one of the most effective means of boosting cardiac function and physical fitness performance in athletes (13). Studies have addressed the chronic impacts of various ET modalities (resistance training and running) on the heart miRNA (14, 15).
There has been an increased interest in a group of non-protein-coding RNAs called microRNAs (miRNAs). MicroRNAs are small non-coding RNAs that have nearly 20 - 25 nucleotides associated with RNA silencing and regulation of gene expression after transcription (16). miRNAs can be found in circulating body fluids, however, the exact physiological effect of circulating miRNAs (cmiRNAs) is unclear (17). Moreover, miRNAs are involved in various main biological processes responsible for negative modulation of gene expression, considered as critical regulatory molecules (18). Regular exercise as a physical replacement treatment is capable of preventing and improving the impacts of estrogen deficiency in the cardiac (19). MicroRNA-133 is involved in cardiac-related (20) and skeletal-related (21) human diseases, which is associated with cardiac disturbances and apoptosis (22). Soy extract supplementation and regular exercise training may also benefit CVD in a menopause rat model (23).
A few studies have also reported the effects of soy IF supplementation and HIIT on the expression of microRNA-133 gene in ovariectomized rats’ hearts. However, these studies do not focus on the combined effects of IF supplementation and HIIT and associated factors caused by ovariectomy. Therefore, we assessed the impacts of HIIT, an IF diet, and the combination of them on the expression of microRNA-133 gene in ovariectomized rats’ hearts.
2. Objectives
We investigate the combined impacts of soy IF supplementation and exercise on the expression of microRNA-133 gene in the heart of ovariectomized rats as a replacement for hormone therapy.
3. Methods
3.1. Animal Care
A total of 50 healthy female Wistar rats (205 g) were purchased from the Animal House of Islamic Azad Universirt, Tabriz branch, Iran. The experimental animals received humane care, and their care followed the international instructions of Laboratory Animals Care’ (NIH Publication No. 85-23, revised 1985).
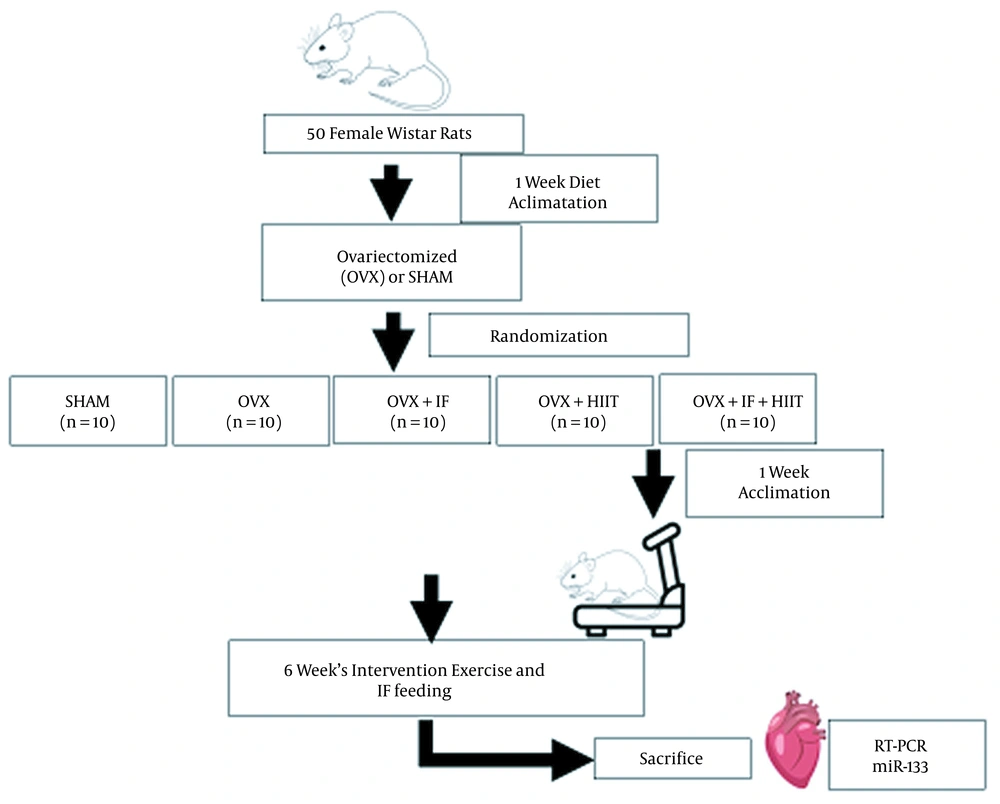
Animals were maintained under controlled conditions (at 22 - 24°C with a light-dark cycle of 12: 12 h) and fed with water and a standard chow diet ad libitum during the experimental procedures. The research followed the policies of the university’s ethics committee (Process No.: 2017/5200690, Date of Approval: February 1, 2017). Following one week of adaptation, animals were assigned to five groups, including sham-operated (SHAM), ovariectomized (OVX), ovariectomized with soy IF supplementation (OVX + IF), OVX with exercise (OVX + HIIT), and OVX with both soy IF and exercise (OVX + IF + HIIT). The experimental rats underwent HIIT (5 days/week) and/or supplied a soy IF supplementation for six weeks. The cardiac microRNA-133 gene expression was analyzed using real-time PCR after six weeks of intervention.
3.2. Ovariectomy Surgery
According to our paper already published elsewhere, the animals underwent a bilateral ovariectomy after one week acclimatization period (23). Then, 50 mg/kg ketamine and 10 mg/kg xylazine was used for anesthetization, and they were subjected to a minor abdominal operation. The oviduct and each ovary were sectioned and separated. Then, a silk thread sutured the skin and muscle walls (23, 24).
3.3. Exercise Training Protocol and Soy Isoflavone Administration
After two weeks recovery period, familiarization of rats in the HIIT group was done with the protocol for a week and they were trained five days per week for six weeks. Rats ran on the treadmill with 90% VO2max for 15 to 30 s and a velocity of 29 m/min in the first week and 36 m/min in the final week. An interval of one minute was considered and the exercise training was repeated five and 12 times in the first and final weeks, respectively. The cooling and warming downtime were five minutes (25). After two weeks of OVX surgery, the rats in OVX + IF and OVX + IF + HIIT groups were fed with 60 mg/kg of soy IF through oral gavage once a day for six weeks on their basal diet. Figure 1 shows the experimental design of this research.
The research experimental design; OVX; ovariectomized, OVX + HIIT, ovariectomized with six-weeks high-intensity interval training (HIIT); OVX + IF, ovariectomized with six-weeks soy isoflavone (IF) supplementation; OVX + IF + HIIT, ovariectomized with six-weeks soy IF supplementation and HIIT
3.4. Molecular Analysis
3.4.1. RNA Isolation and the cDNA Synthesis
After sacrificing the animals, their hearts were excised after exercise training for six weeks (16). Then, extraction of messenger RNA (mRNA) and miRNA was done from the heart left ventricle respectively through the RNX-Plus solution kit (Fermentase, Germany) and mir-amp kit (Parsgenome Co. Iran) considering the manufacturer’s guidelines (by chloroform layer separation and then receiving ethanol and isopropanol). A260/280 ratio and RNA quantity were assessed by the ND-1000 (Thermo Fisher Scientific, Waltham, MA), and gel electrophoresis stained with GelRed (Biotium, Hayward, CA, USA) was applied for assessing the sample integrity. The microRNA-133 gene expression was quantitatively assessed by RT-PCR (Bioneer Corporation, Korea). Primer sequences were determined using Primer3Plus (http://www.bioinformatics.nl) and Primer-BLAST (http://www.blast.ncbi.nlm.nih.gov) (Table 1). Normalization of the PCR products was done for internal control housekeeping gene-3-phosphate dehydrogenase (GAPDH). Then, reverse transcription of total RNA (1 μL) was done using DNase I (1 μL), RiboLock RNase‐inhibitor (0.25 μL), Revert Aid MMuLV reverse transcriptase (1 μL), dNTPS (2 μL), and random hexamer primers (1 μL), at 25°C for 10 min, and then 60 min at 42°C in a final volume of 20 μL to synthesized cDNA from the mRNA template. The reaction was finalized through heating at 70°C for 5 min. Moreover, cDNA generation from the mRNA sample was done using the miR-Amp kit (Parsgenome Co. Iran) as instructed (19).
| Gene | Accession Number | Primer Sequence a |
|---|---|---|
| MicroRNA-133 | NM-126426 | F: 5’-ACACTCCAGCTGGGTTTGGTCCCCTTCAAC-3’; R: 5’- AATTCAGTTGAGCAGCTGGT-3’ |
| GPDH | NM-204162 | F: 5’-CTCGCTTCGGCAGCACA-3’; R: 5’-AACGCTTCACGAATTTGCGT-3’ |
a Obtained from NCBI (www.blast.ncbi.nlm.nih.gov)
3.4.2. Quantitative Real-time PCR (RT-qPCR)
Quantitative PCR (qPCR) was done following the procedure of Habibi et al. (19). Then, a master mix with 12.5 μL SYBRGreen PCR Master Mix (Fermentase, Germany), 2 μL reverse primer, 2 μL forward primer, and 8.5 μL water in a final volume of 25 μL were provided to conduct real‐time PCR. After that, 2 μL of reverse-transcribed cDNA was mixed with the PCR master mix and a final volume of 25 μL was achieved. In addition, a negative control was considered in each run through the elimination of the cDNA specimen in the tube to check the amplification accuracy. The PCR protocol was applied in the real‐time PCR System (Applied Biosystems, USA) in three stages, as follows:
(1) Initial denaturation (95°C /10 min); (2) amplification in three steps (15 s / 95°C and then 30 s at 58°C for Bcl‐2 and 30 s at 60°C for Mir‐133 gene; and 30 s at 72°C) considering the repetition for 40 times; and 3‐melting curve analysis (one cycle: 72 - 95°C; temperature transition rate: 1°C/sec for 5 sec). These stages were done for three times. Monitoring of real-time quantification was done through the measurement of an elevation in fluorescence due to the attachment of the SYBR Green dye to double‐stranded DNA after each cycle. The mRNA relative quantification related to each considered gene was determined according to its threshold cycle (Ct) than the Ct of GAPDH as the reference gene using the 2ΔΔct method. PCR specificity reaction was approved using the melting curve analysis and then gel electrophoresis, stained with GelRed (Biotium, California).
3.5. Statistical Analysis
The statistical data were presented as mean (SD) error. Data analysis was done by the Shapiro-Wilk test, which approved the data Gaussian distribution. One-way ANOVA and then Tukey’s test used analyzed the differences among the groups. All analyses were carried out by IBM SPSS 20 and at a significance level of < 0.05.
4. Results
The OVX group showed a significantly higher bodyweight following six weeks than the SHAM group (P < 0.05) (Table 2). Soy IF and/or exercise (HIIT+ IF) could return bodyweight to baseline due to no difference between these groups and SHAM. Ovariectomized + HIIT, OVX + IF, and OVX + HIIT +IF groups had different body weights compared to the OVX group. Dietary intake did not differ significantly between the groups.
| Groups | SHAM | OVX | OVX + IF | OVX + HIIT | OVX + IF + HIIT |
|---|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 | 10 |
| FinalBW (g) | 278.2 ± 2.1 | 295.3 ± 3.5 b | 277.4 ± 2.5 | 279.2 ± 1.8 | 278.5 ± 3.2 |
| Food Intake (g/day) | 17.43 ± 2.46 | 16.51 ± 1.9 | 17.33 ± 3.21 | 16.41 ± 2.21 | 17.18 ± 3.42 |
Abbreviations: SHAM, sham-operated; OVX, ovariectomized group; BW, body weight; OVX + HIIT, ovariectomized with eight-week high-intensity interval training group; OVX + IF, ovariectomized with six-weeks soy isoflavone supplementation group; OVX + IF + HIIT, ovariectomized with six-weeks soy isoflavone supplementation and HIIT group.
a Values are means ± SEM (n = 10).
b P < 0.05 comparison between SHAM and other groups.
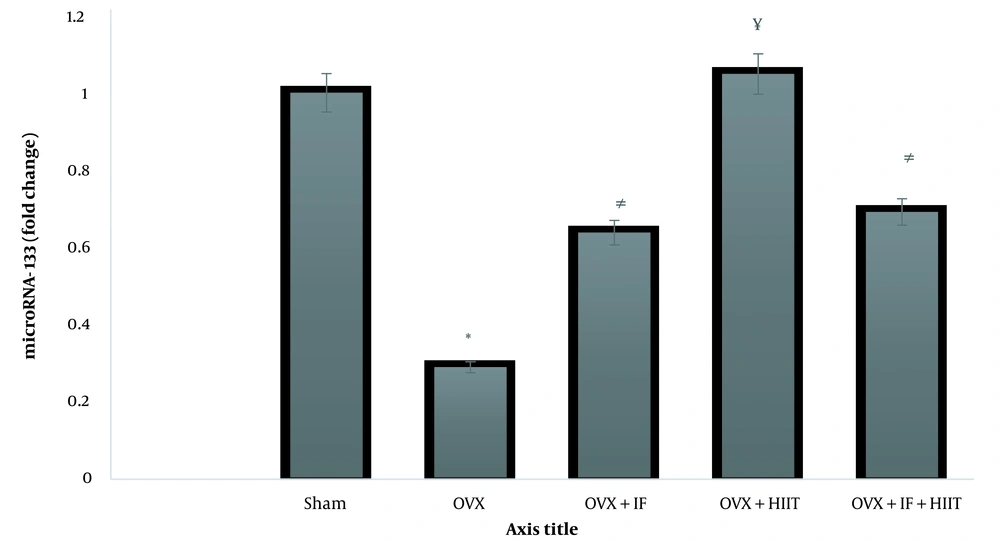
The heart microRNA-133 expression level is displayed in Figure 2, which was significantly lower in the heart of OVX compared to the SHAM groups (P < 0.05). In addition HIIT, IF and HIIT+ IF groups increased expression of microRNA-133 gene than OVX animal group (P < 0.05).
Effect treatments on the expression level of microRNA-133 gene in the heart; OVX, ovariectomized group; OVX + HIIT, ovariectomized with six-weeks high-intensity interval training (HIIT) group; OVX + IF, ovariectomized with six-weeks soy isoflavone (IF) supplementation group; OVX + IF + HIIT, ovariectomized with six-weeks soy IF supplementation and HIIT group. Data indicate mean ± SEM (n = 10). * Significant difference than the sham-operated (SHAM) (P < 0.01). # Significant difference than the SHAM and OVX (P < 0.05). ¥ Significant difference than the OVX, OVX + IF, OVX + IF + HIIT (P < 0.05)
5. Discussion
Many cardiovascular events develop in postmenopausal women. Heart disease occurs in females on average ten years later than males, which is associated with the protective effects of female sex hormones, especially estrogens, before menopause (26). We evaluated the effect of six weeks of HIIT and soy IF treatment on the microRNA-133 expression in ovariectomized rat’ heart. It was hypothesized that ovariectomy van induce apoptosis and a pathological cardiac gene expression pattern. In addition, the HIIT training and soy IF administration alone or in combination would lead to upregulation of cardiac anti‐apoptotic gene.
Down‐regulation of microRNA‐133 cardiac expression in ovariectomized animals was linked to anti‐apoptosis compared to the SHAM surgery rats. Six weeks of HIIT training increased the expression of the microRNA‐133 gene. The expression of the microRNA‐133 gene increased by eight weeks of soy IF administration. Combining soy IF supplementation and regular HIIT training has no significant cardiac protective effect compared to employing either strategy alone. Our results suggested that the ovariectomy model successfully reduced microRNA-133 expression, indicating an association between rodent vascular function and such menopause model. These results are consistent with those of (19, 23).
The mean weight of the ovariectomized rats increased significantly compared to the SHAM group. Our findings are in line with those found in previous reports showing that weight gain in postmenopausal women is connected to estrogen deficiency, increased visceral fat, and inflammation of metabolic disorders (27-29). This process occurs through interference with leptin, which is known as a hormone for producing adipose cells and plays a vital role in regulating body weight and appetite (30). Studies have shown that estrogen supplementation reduces food intake and body weight after menopause. The estrogen receptor alpha has adjusted the effect that has been proposed. Additionally, estrogen plays an essential role in reducing body composition through the reduced accumulation of fatty acids, triglyceride synthesis, and lipogenesis (31).
Isoflavones are phytochemicals present in many legumes as a large group of non-steroidal materials with various structures obtained from plants attached to estrogen receptors (ERs) in humans and animals. Isoflavones exhibit have more tendency for ER-A compared to ER-> and have estrogen antagonist and estrogen-agonist characteristics. The biochemical genistein, glycitein, formononetin daidzein, and biochanin A are some IFs. Daidzein and genistein are highly found in soybeans and soy products and red clover, kudzu, and the American groundnut (32). Recent systematic review and meta-analysis suggested that soy IFs decreased serum total cholesterol levels in postmenopausal women (33).
In the present investigation, microRNA-133 was markedly down‐regulated in cardiomyocytes from OVX rats’ heart compared to the SHAM group. Such findings are consistent with previous reports (19, 34), which states that ovariectomy down‐regulated Mir‐133 expression levels in the cardiac tissue. The OVX-induced increase in genes that mediate inflammation (IL-6, inhibin beta, TNF-alpha, SOCS3, and SOCS2), an OVX-induced reduction in the myocardial mRNA expression of genes are associated with regulating vasodilation (soluble guanyl cyclase and endothelial NOS), an OVX-related elevation in extracellular matrix genes (alpha1, collagen12, connexin 43), and an OVX-induced elevation in proapoptotic genes (calpain and caspase 3).
We also showed that HIIT improve the expression of microRNA-133 in ovariectomized rats’ heart. Interestingly, HIIT has a more significant cardio protective effect than the other treatment groups. MicroRNAs are mediators of processes involved in adaptation to exercise training, such as cardiac (35) and skeletal muscle (36). Also, vastus lateralis of healthy individuals were found with significant up-regulation of hsa-miR-133a and hsa-miR-1 expression levels against cycle ergometer exercise bout at 65% of Pmax (37). In addition, a 12-week aerobic exercise on a cycle ergometer decreased the hsa-miR-133a, hsa-miR-1, hsa-miR-206and hsa-miR-133b. Greater microRNA-133 activity in cardiac muscle of rat models reduced caspase‐3 and caspase‐9 with H2O2 (38), decreased malondialdehyde (MDA) and reactive oxygen species (ROS) levels and increased glutathione peroxidase (GPx) levels and superoxide dismutase (SOD) activity to protect the heart against apoptosis (34). We showed the soy IF supplementation as a probable modality for decreasing ovariectomy-related alterations in this cardiac gene expression. Also, six weeks of soy IF administration increased the expression of microRNA-133 in ovariectomized rats.
These findings were consistent with the results obtained by other human-based studies, based on which soybean IF administration could benefit lipid metabolism and aerobic exercise performance (39) and cardiovascular disease risk factors in menopausal female cases by the modulation of hepatic protein expression profiles (40). Furthermore, previous studies have shown that soy IF supplementation increase cardiometabolic benefits for postmenopausal women and activates peroxisome proliferator-activated receptors (PPARγ)-related signal transduction pathways, modulating adipogenesis, lipogenesis, and lipolysis (41). Isoflavones have anti-estrogenic and estrogenic activities, associated with their level, the level of endogenous sex hormones, and the involved organ. The present could not show a more significant cardiac protective effect of combining soy IF supplementation and regular HIIT than employing either strategy alone. Future study should determine whether our investigations on microRNA-133 expression translate into assessable physiological impacts on cardiac function, especially skeletal muscle.
5.1. Conclusions
The results supported the beneficial effects of HIIT and/or soy IF administration on the microRNA-133 actions to enhance the cardiac function. Furthermore, we could speculate that HIIT administration can provide an effective cardioprotective in a menopause rat model than soy supplementation or their combination.