1. Background
Transdermal delivery relies on drug absorption through the skin. In addition to controlled and continuous drug delivery, transdermal drug delivery offers several advantages, such as first-pass intestinal and hepatic bypass, the avoidance of gastrointestinal irritation, which is commonly associated with oral medications, and easier drug localization at the target site (1). According to this study, natural ingredients can potentially improve medication penetration through the skin by reversibly lowering skin barrier resistance. The current study begins with an overview of the current state of natural compounds based on SAR studies showing considerable reactivity increase (1). Supersaturation is a physical improvement approach in which the thermodynamic activity is increased beyond the saturation level. In addition to being low-cost, this method does not compromise skin integrity (1). Stratum corneum partitioning and dispersion, transit into dermis, and penetration into deeper tissues are critical phases of skin permeation (2).
Chemical and physical penetration enhancers allow high drug concentrations to enter the systemic circulation or deeper tissues (1). Piroxicam and its package formula C15H13N3O4S are non-steroidal anti-inflammatory drugs beneficial in treating fever, pain, and inflammation (2). By inhibiting cyclooxygenase, this medicine reduces prostaglandin synthesis and declines inflammation and pain (2). Recent research used differential scanning calorimeter (DSC) and FTIR to study lipid arrangement and skin structure (FT-IR).
2. Methods
2.1. Materials
Piroxicam was given by Ramopharmin (Tehran, Iran). Barij Essence, an Iranian company, supplied eucalyptus oil, olive oil, clove oil, and sunflower oil. Merck, Germany, provided sesame oil, sodium phosphate monobasic, and sodium phosphate dibasic, and our laboratory-produced deionized water (3).
2.2. Animal Studies
Male Wistar rats weighing 200 to 250 g underwent ether-anesthesia in vitro permeation testing. A razor and electric shaver removed head hair.
The skin on the abdomen was cut off, and extra stratum corneum fat was removed from the surrounding area.
2.3. Piroxicam Assay
At a wavelength of max = 362 nm, the quantity of piroxicam was measured using the UV spectroscopic technique (1).
2.3.1. In Vitro Permeation Study
For permeation studies, diffusion cells with effective areas of 4.902 cm2 and receptor compartments of 40 mL were used. Prior to usage, the skin samples were hydrated and positioned with the epidermis towards donor media between donor and receptor cell compartments.
Piroxicam (0.5%, w/v) solution was mixed with distilled water in the donor compartment. The donor chamber was filled with piroxicam, and the receptor chamber was filled with phosphate-buffered saline (PBS) (pH: 7.4).
Diffusion cells were heated in a magnetic stirrer bath at 37°C. The water bath was 37°C, and the receptor media was agitated at 200 rpm.
A sample of 2 mL of the receptor media was obtained at intervals of 0.5, 1,…, 7, and 24 hours, and an equal volume of fresh buffer was then added (4).
2.4. Data Analysis
Piroxicam was calculated and plotted based on the area of the diffusion surface as a function of how long it took to reach the receptor. The linear parts of the slope of the permeation curve were used to get the steady-state flux (mg/cm2.h). Kp, cm/h, was found by using the Equation 1 (2).
Where Jss is the steady-state flux and Cv is the initial concentration of piroxicam in the receptor compartment. Enhancement ratios (ER) were obtained using Equation 2.
2.4.1. Differential Scanning Calorimeter
According to a DSC system was used, the penetration enhancers changed the structure of the whole skin. These was placed in chemical penetration enhancers for 4 hours after the skin was totally hydrated. We removed the additional penetration enhancers and sealed the samples to stop leakage.
6 to 10 milligrams of skin were roughly sliced, placed in an aluminum pan, and sealed. The reference was a pan that was empty.
The samples were heated up to 200°C at a rate of 5°C per minute several times. DSC analyzers were calibrated with indium three times (5).
2.4.2. FT-IR Studies
The skin samples had to be vacuum dried for 30 minutes at 650 mm Hg and 25°C to remove any traces of the penetration enhancers. Then, the samples were desiccated to remove any residual penetration enhancers. The samples were scanned using the Uker FT-IR between the wavelengths of 4000 and 500 cm-1 (6).
3. Results
3.1. Effect of Enhancers on Piroxicam Skin Permeability
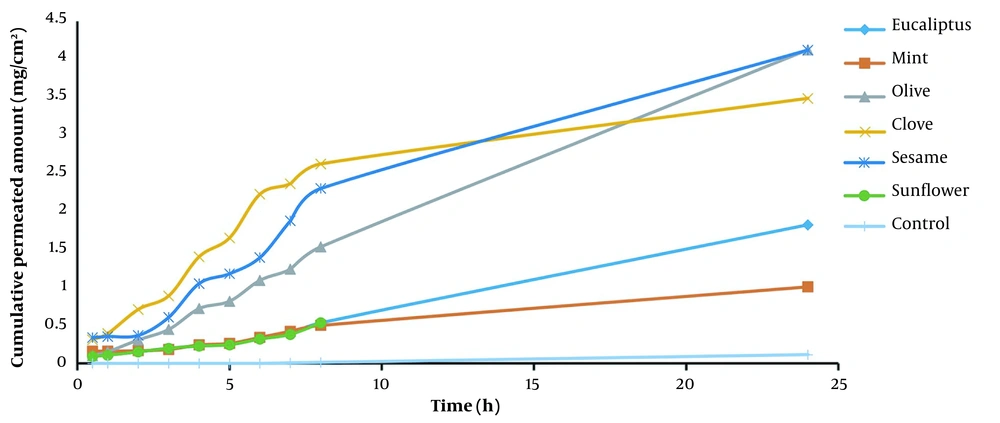
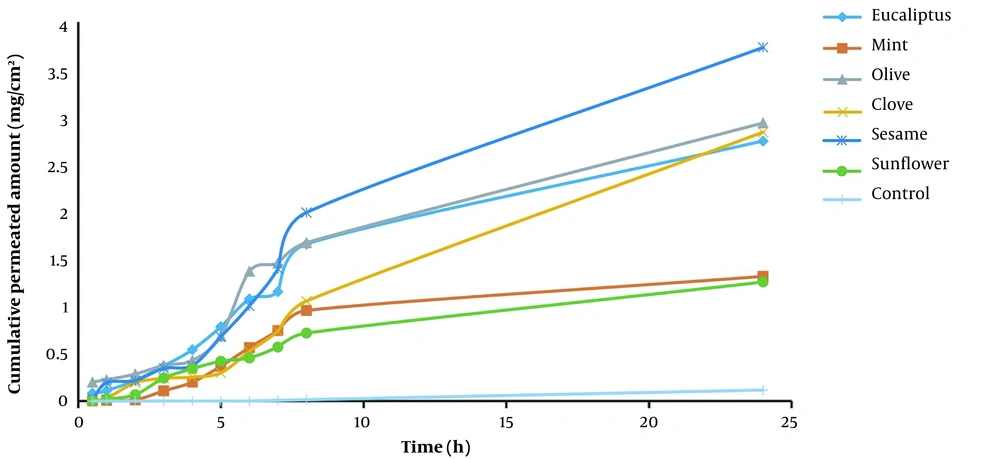
Tables 1 and 2 and Figures 1 and 2 demonstrate how much piroxicam entered the body after using each enhancer. ERD is the ratio of drug diffusion before and after the enhancer in comparison to the control, and ERflux is the ratio of drug flux before and after exposure to the enhancer (7). An enhancer-free control was used to compare hydrated skin to the control. Olive oil increased piroxicam flux by 0.443-fold compared to the control. Olive oil increased more than sesame oil, eucalyptus oil, clove oil, menthol oil, and sunflower oil (0.122 fold). After treatment, the diffusion coefficient was changed differentially by each enhancer.
| Enhancer | Jss (mg/cm2.h) | Dapp (cm2/h) | P (cm/h) | Tlag (h) | ERflux | ERD | ERp |
|---|---|---|---|---|---|---|---|
| Control | 0.021 ± 0.01 | 0.0790 ± 0.06 | 0.0030 ± 0.0005 | 1.4 ± 0.4 | - | - | - |
| Eucalyptus oil | 0.199 ± 0.07 | 0.0396 ± 0.0014 | 0.0398 ± 0.014 | 1.64 ± 0.76 | 13.91 ± 0.69 | 0.517 ± 0.17 | 212.44 ± 0.8 |
| Mint oil | 0.1619 ± 0.048 | 0.0646 ± 0.008 | 0.0323 ± 0.0097 | 2.85 ± 0.08 | 11.84 ± 1.58 | 1.4582 ± 0.03 | 184.3 ± 0.8 |
| Olive oil | 0.4439 ± 0.13 | 0.0528 ± 0.0013 | 0.0887 ± 0.006 | 3.9 ± 1.2 | 27.23 ± 1.1 | 1.2192 ± 0.8 | 389.84 ± 8.0749 |
| Clove oil | 0.1915 ± 0.06 | 0.0833 ± 0.01 | 0.0383 ± 0.01 | 2.97 ± 0.08 | 13.73 ± 1.5 | 1.9374 ± 0.6 | 212.16 ± 8.8 |
| Sesame oil | 0.263 ± 0.01 | 0.0605 ± 0.05 | 0.0526 ± 0.02 | 2.24 ± 0.9 | 15.32 ± 1.8 | 1.3738 ± 0.47 | 212.96 ± 10.46 |
| Sunflower oil | 0.1225 ± 0.09 | 0.2146 ± 0.03 | 0.0245 ± 0.01 | 5.98 ± 0.7 | 6.21 ± 1.15 | 5.1688 ± 1.2 | 78.83 ± 8.4 |
The Permeability Parameters After Pretreatment with Permeation Enhancers Compared with Control (2 Hours) (n = 3) a
| Enhancer | Jss (mg/cm2.h) | Dapp (cm2/h) | P (cm/h) | Tlag (h) | ERflux | ERD | ERp |
|---|---|---|---|---|---|---|---|
| Control | 0.021 ± 0.01 | 0.0790 ± 0.06 | 0.0030 ± 0.0005 | 1.41 ± 0.4 | - | - | - |
| Eucalyptus oil | 0.644 ± 0.3 | 0.085 ± 0.007 | 0.1288 ± 0.06 | 2.67 ± 0.2 | 36.70 ± 1.5 | 1.97 ± 0.50 | 503.6 ± 11.45 |
| Mint oil | 0.0816 ± 0.02 | 0.1236 ± 0.04 | 0.0163 ± 0.004 | 3.42 ± 1.3 | 5.08 ± 0.3 | 2.93 ± 0.68 | 73.42 ± 61.62 |
| Olive oil | 0.2190 ± 0.07 | 0.0621 ± 0.03 | 0.0438 ± 0.01 | 1.65 ± 0.15 | 13.34 ± 1.05 | 1.4 ± 0.12 | 190.42 ± 2.47 |
| Clove oil | 0.4757 ± 0.01 | 0.0197 ± 0.01 | 0.0951 ± 0.005 | 1.6 ± 0.3 | 32.68 ± 8.8 | 0.37 ± 0.32 | 495.07 ± 19.95 |
| Sesame oil | 0.7367 ± 0.06 | 0.0310 ± 0.0030 | 0.1473 ± 0.09 | 1.27 ± 0.6 | 53.82 ± 4.01 | 0.56 ± 0.32 | 837.74 ± 10.01 |
| Sunflower oil | 0.0664 ± 0.03 | 0.0523 ± 0.004 | 0.0133 ± 0.006 | 2.47 ± 0.6 | 3.91 ± 0.32 | 1.12 ± 0.44 | 54.73 ± 4.54 |
The Permeability Parameters After Pretreatment with Permeation Enhancers Compared with Control (4 Hours) (n = 3) a
Sunflower oil had the highest effect (0.214 fold), followed by clove oil (0.083 fold), menthol oil (0.064 fold), sesame oil (0.060 fold), olive oil (0.052 fold), and eucalyptus oil (0.039 fold). There was no significant difference in the permeability coefficient (P) after treatment. Based on these findings, it can be concluded that these enhancers mostly assisted the medications in penetrating the skin and worked by creating a liquid pool in the stratum corneum and destroying its lipid structure (8).
3.2. Differential Scanning Calorimetry
The thermotropic behavior of the treated skin was examined using the mean transition temperature (Tm) and their enthalpies (H).
Table 3 shows the temperatures and enthalpies. The Tm1 and Tm2 temperatures of hydrated rat skin were 67.5 and 112 degrees celsius, indicating that lipids had melted and intracellular keratin had been permanently degraded. The decrease in Tm may be attributable to the disintegration of lipids in the bilayer and irreversible protein degradation in the SC.
| Enhancer | Penetration Enhancer | Transition Temperature (°C) | Transition Enthalpy (mj/mg) | |
|---|---|---|---|---|
| Tm1b | Tm2c | ΔH1d | ΔH2e | |
| Water (control) | 67.5 ± 1 | 112 ± 3.4 | -7.01 ± 0.4 | -551.35 ± 9 |
| Menthol oil | 38 ± 0.2 | 111 ± 0.1 | 0.721 ± 0.01 | 1.865 ± 0.4 |
| Eucalyptus oil | 31 ± 0.4 | 127.5 ± 1.1 | 2.672 ± 0.1 | 8.866 ± 0.8 |
| Olive oil | 37.7 ± 0.9 | 11.5 ± 0.9 | 5.844 ± 0.66 | 6.118 ± 0.2 |
| Sesamoid | 33 ± 0.2 | 119 ± 0.2 | 0.45 ± 0.01 | 5.612 ± 0.4 |
| Clove oil | 36 ± 0.2 | 116 ± 0.5 | 0.906 ± 0.02 | 2.204 ± 0.1 |
| Sun flower oil | 23.1 ± 0.2 | 118 ± 0.8 | 0.85 ± 0.4 | 2.224 ± 0.3 |
The Effect of Penetration Enhancer on the Thermal Properties of the Rat Skin (n = 3) a
Lipid bilayers and protein-lipid complexes decrease enthalpy when lipids move around, but this is not always the case (9). Tm1 shifted in lipid forms from lamellar to disordered red according to Kaushik and Michniak-Kohn (9). There were three endothermic transition peaks at temperatures of 59 - 63°C (Tm1), 75 - 82°C (Tm2), and 99.5 - 120°C (Tm3). It was believed that a shift in protein-lipid forms was the root of Tm2 (10). Alternatively, the polar head groups of lipids and Tm3 are thought to be broken during irreversible denaturation, respectively (11).
There was a rise in Tm2 and Tm1 when it was compared to hydrated rat skin. Tm1 was not found after treatment with eucalyptus oil. Eucalyptus oil might be helpful in this situation. In the DSC results, skin were pretreated with menthol, sesame oil, clove oil, olive oil, and sunflower oil has lower Tm1, Tm2, and ΔH1 and ΔH2 than rat skin, which has not been pretreated at all. The lipid layer in the bilayer and the irreversible protein layer in the SC layer were broken down by menthol oil and other oils in this study. As a result, these oils can enhance the absorption of oil into bodies in multiple ways.
The Tm1 of the skin decreased when olive oil was applied before it was hydrated. Tm1, ΔH1, and ΔH2 went up compared to the hydrated skin value. Hence, olive oil can make the skin more permeable by breaking down lipids in the bilayer and proteins in the SC layer (10).
4. Discussion
4.1. FT-IR Spectroscopy
Tables 4 - 6 show changes in the position of peaks and the intensity of their peaks. When the wave number shifts to a higher wave number (blue shift), the SC membrane (lipid bilayer) becomes more fluid. Consequently, the barrier properties of the SC might were broken down, permitting the substance to pass through faster (12).
| Enhancer | Asymmetric C-H Stretching | Symmetric C-H Stretching | C=O Stretching of Lipid Ester | |||
|---|---|---|---|---|---|---|
| Wave Number | %D | Wave Number | %D | Wave Number | %D | |
| Water (control) | 1.8355 ± 0.008 | - | 1.95 ± 0.005 | - | 2.061 ± 0.001 | - |
| Mint oil | 0.455 ± 0.006 | 75.21 | 0 | 100.00 | 0 | 100.00 |
| Eucalyptus oil | 0.517 ± 0.01 | 71.83 | 0.436 ± 0.008 | 77.64 | 0.775 ± 0.006 | 62.40 |
| Olive oil | 0.381 ± 0.001 | 79.24 | 0.207 ± 0.01 | 89.38 | 0.425 ± 0.002 | 79.38 |
| Sesame oil | 1.792 ± 0.007 | 2.37 | 1.819 ± 0.009 | 6.72 | 1.522 ± 0.01 | 26.15 |
| Clove oil | 1.227 ± 0.004 | 33.15 | 0.464 ± 0.01 | 76.21 | 0 | 100.00 |
| Sun flower oil | 2.278 ± 0.01 | -24.11 | 2.114 ± 0.005 | -8.41 | 1.956 ± 0.007 | 5.09 |
| Enhancer | C-H Stretching Asy | C-H Stretching Sym | C=O Stretching of Lipid Ester | Amide І | Amide ІІ |
|---|---|---|---|---|---|
| Water (control) | 0.16 ± 2918.77 | 2856.34 ± 0.16 | 0.14 ± 1731.68 | 1667.04 ± 0.12 | 1547.67 ± 0.11 |
| Mint oil | 2950.04 ± 0.11 | - | - | 1660.28 ± 0.12 | 1552.69 ± 0.16 |
| Sesame oil | 2904.35 ± 0.18 | 2804.55 ± 0.11 | 1746.61 ± 0.19 | 1685.61 ± 0.12 | 1576 ± 0.10 |
| Sun flower oil | 2890.26 ± 0.18 | 2830.94 ± 0.14 | 1745.01 ± 0.17 | 1667.22 ± 0.15 | 1571.20 ± 0.13 |
| Clove oil | 2981.66 ± 0.15 | 2915.86 ± 0.11 | - | 1690.2 ± 0.21 | 1642.65 ± 0.17 |
| Eucalyptus oil | 2848.59 ± 0.12 | 2741.57 ± 0.15 | 1791.51 ± 0.13 | 1654.93 ± 0.18 | 1565.24 ± 0.12 |
| Olive oil | 2990.78 ± 0.12 | 2887.85 ± 0.14 | 1743.31 ± 0.18 | 1626.18 ± 0.18 | 1549.92 ± 0.15 |
FT-IR Peak Wave Numbers (cm-1) Change Compared to Control (Untreated Skin) and Abdominal Hydrated Whole Skin Rat After Treatment with Different Enhancers (n = 6) a
| Enhancer | C-N Stretching of Keratin | C-N Stretching of Keratin | ||
|---|---|---|---|---|
| Peak Height | Wave Number | %D | Wave Number | %D |
| Water (control) | 2.111 ± 0.006 | 0.00 | 2.151 ± 0.005 | - |
| Mint oil | 0.731 ± 0.01 | 65.37 | 0.644 ± 0.005 | 70.06 |
| Eucalyptus oil | 0.727 ± 0.005 | 65.56 | 0.727 ± 0.007 | 66.20 |
| Olive oil | 0.479 ± 0.01 | 77.31 | 0.611 ± 0.01 | 71.59 |
| Sesame oil | 1.414 ± 0.008 | 33.02 | 1.518 ± 0.01 | 29.43 |
| Clove oil | 1.889 ± 0.02 | 10.52 | 1.94 ± 0.006 | 9.81 |
| Sun flower oil | 1.961 ± 0.007 | 7.11 | 1.888 ± 0.009 | 12.23 |
The Decrease in Mean Peak Height (± SD) Compared with Control (Hydrated Skin) of C=O Stretching (Amide I) and C-N Stretching of Keratin (Amide II) and the Absorbance of Abdomen Hydrated Whole Skin Rat After Treatment with Different Enhancers (n = 3) a
Peak height and wave number changes are seen in the FT-IR spectrum of skin, which has been sprayed with menthol oil. A blue shift in the height of peak number (2950.04 cm-1) was observed in the skin, which had been treated with menthol, meaning that proteins in the SC layer were irreversibly broken down. Skin pretreated with menthol oil showed a red shift at the wave number (1660.28 cm-1). Therefore, the lipid groups in the skin were rearranged, making the SC barrier stronger. Rat skin, which has been treated with sesame oil presents changes in peak height and wave number. There were redshifts on the treated skin with the same oil at the wave number (2904.35 cm-1). Therefore, the lipid groups were rearranged and made the SC barrier stronger. Sesame oil pretreated skin exhibited a blue shift in peak height (1746.61 cm-1). In other words, the proteins in the SC layer were irreversibly broken down. A red shift was observed on skin, which had been treated with sunflower oil at the wave number (2890.26 cm-1). Hence, the lipid groups made up the skin's barrier properties were rearranged. People who used sunflower oil on their skin saw a blue shift appearing its taller at the peak number (1745.01 cm-1). Skin treated with clove oil showed a blue shift in the height of the peak number (2981.66 cm-1). Therefore, proteins in the SC layer had been irreversibly broken down, and wave numbers had changed. A blue shift was seen in pretreated skin, revealing the peak number (1690.2 cm-1).
Eucalyptus oil made the skin red at wave number (2848.59 cm-1), meaning that the lipid groups in the skin was moved around and made it more resistant to water. Pre-eucalyptus oil-treated skin showed a blue shift in the height of peak number (1791.51 cm-1), and proteins in the SC layer were irreversibly denatured. The red shift was seen at wave number (1654.93 cm-1), meaning that lipid groups were reoriented, making the SC barrier stronger. The graph illustrates the change in peak height and wave number of olive oil-treated rat skin over time.
4.2. Conclusions
According to the results, the applied penetration enhancers increased the drug's ability to pass through the skin of rats. Several different mechanisms, such as lipid fluidization, disruption of lipid structure, and also intracellular keratin irreversible denaturation in stratum corneum by sesame oil, menthol oil, clove oil, eucalyptus oil, sunflower oil, and olive oil, were the primary probable mechanisms for higher ERflux, ERD, and ERP ratios in comparison with water-hydrated skin.