1. Background
Some studies have suggested that the acute effects of exercise can induce programmed cell death (apoptosis) in various tissues (1-3). Evidence indicates that exercise-induced apoptosis mechanisms are related to neuro-endocrine system stimulations (catecholamines, cortisol), free radical species, production of reactive oxygen species (ROS), and cytokine (i.e., tumor necrosis factor-α (TNF-α), interleukin-6, etc.) levels, which can influence several extracellular and intracellular signaling pathways (4).
Apoptosis mainly consists of two main pathways, including the extrinsic pathway and the intrinsic pathway. Mitochondria play an essential role in the mechanism of apoptosis, when mitochondria are exposed to apoptotic stimuli, cytochrome c is released from the mitochondria to the cytoplasm (5). Cytochrome c appears to be primarily mediated by direct or indirect ROS function. Bax protein oligomerizes and forms pores on the surface of the outer mitochondrial membrane to release cytochrome c (6). This cytochrome c promotes Apaf-1 in dATP or ATP, apoptosome formation, and caspase-9 activation (4). Activated caspase-9 activates caspase-3, causing apoptotic cell death (5). The Bcl-2 (B-cell lymphoma 2) regulates apoptosis by inhibiting cytochrome c release from the mitochondria (4).
Besides mechanical damage to cytoskeletal proteins, eccentric exercise induces more significant apoptosis and oxidative stress than concentric exercise (7). Various types of eccentric exercise evoked an increased circulating concentration of cytochrome c, caspase-9, caspase-3, and Bax (1, 2, 8). Bax and caspase-3 mRNA were significantly increased after a single bout of eccentric exercise in rat skeletal muscle (9). Circulating levels of caspase-3 increased significantly after a bout of intense resistance exercise in untrained subjects (3). Furthermore, resistance exercise has been shown to elevate serum caspase-9 in athletes (2). Controlling cellular balance during acute exercise is essential to preventing excessive or unexpected apoptotic cell death (10). Therefore, antiapoptotic therapy in normal cells may benefit those involved in moderate to severe exercise.
The benefits of berries over other fruits include vitamins, minerals, dietary fiber, and fatty acids, as well as polyphenolic phytochemicals (phenolic acids, flavonoids, tannins, and lignans) (11). Among the popular sources of dietary berries, strawberries are rich in polyphenols, especially anthocyanins, ellagic acid, and ellagitannins, which have antioxidant and anti-inflammatory properties and prevent oxidative stress-related diseases, obesity, metabolic syndrome, cardiovascular diseases, some cancers, and neurological diseases (11-14). No study has been conducted to evaluate the effects of strawberry supplementation on exercise-induced apoptosis.
2. Objectives
The current study aimed to investigate the results of the strawberry extract on resistance exercise-induced apoptosis in untrained healthy women considering the protective antioxidative property of strawberries, protective effects against free radicals (11), oxidative stress (12), DNA damage (15), and proinflammatory cytokines (16). This hypothesis was tested in the present study to examine whether strawberry supplementation can attenuate systemic markers of exercise-induced apoptosis.
3. Methods
3.1. Subjects
In a randomized, double-blind, placebo (PL)-controlled crossover study, sixteen subjects were matched based on age and maximum strength and randomly divided into two groups of the strawberry extract supplementation (SES) or the PL. Only untrained females (early follicular phase of the menstrual cycle) were recruited due to the possible effect of gender on exercise-induced apoptosis (17). In addition, it seems that trained subjects with high fitness levels experienced a low level of oxidative stress and subsequently encountered less exercise-induced apoptosis (3). Table 1 shows the physiological characteristics of the study subjects. The subjects who were free of any chronic diseases used any medication/androgens or nutritional supplements for the preceding six months, tobacco products and antibiotics taken eight weeks before, supplements containing polyphenols, consuming foods with high polyphenols, or adverse reactions to strawberries.
| Variables | Strawberry Extract, Mean ± SD | Placebo, Mean ± SD | P-Value |
|---|---|---|---|
| Age, y | 24.7 ± 4.6 | 23.5 ± 3.2 | 0.17 |
| Weight, kg | 68.9 ± 8.7 | 66.6 ± 7.4 | 0.21 |
| Height, cm | 163.3 ± 4.8 | 164.4 ± 4.2 | 0.23 |
| BMI, kg/m2 | 25.9 ± 5.2 | 24.7 ± 4.6 | 0.18 |
| Fat, % | 20.4 ± 3.7 | 20.1 ± 4.3 | 0.24 |
3.2. Preparation of Extract of Strawberry
Ripe strawberries were collected from the farm, cut into small pieces, and dried in shadow to extract the preparation. The strawberries were utterly crushed, and water solvent was used to prepare the phenolic extract after cleaning at ambient temperature and removing impurities. The resulting solution was kept in the dark, closed environment for 24 hours at room temperature. After wiping with Whitman No. 1 filter paper, the powder was extracted by the Heidolph (Germany) vacuum operator (45°C and 270 r/min) using a vacuum funnel. Subsequently, all existing water was removed by freeze-drying (-70°C), thoroughly dried, and powdered. Then, the strawberry extract was stored at 22 - 25°C under sterile conditions for other measurements.
3.3. Pilot Experiment
The administration of strawberry extract supplementation (100 mg/day for 14 days) decreased inflammatory markers after acute exercise in overweight women (18). However, no human study has provided consistent information for identifying a minimum dose to attenuate serum apoptosis markers following acute exercise. Therefore, a pilot test (n = 8) was performed to compare the effect of three doses of strawberry extract (300, 500, and 700 mg) against PL to identify an optimal dose required to lower serum apoptosis markers. In this pilot experiment, the 300 mg/day dose did not significantly attenuate caspase-3 at 14 days following strawberry extract supplementation compared to the placebo (15%; effect size = 0.14, 95% confidence interval [-0.06 – 0.79]). Caspase-3 attenuating with the 500mg/day dose was significantly less (19%) with a small effect size (0.27; 95% confidence interval [-0.13 – 0.63]). The strawberry extract dose of 700mg/day resulted in at least a 28% attenuating serum caspase-3 (effect size = 0.49; 95% confidence interval [0.07 – 0.95]). Therefore, a 700 mg/day dose was selected for the present research design.
3.4. Muscle Strength Testing and Familiarization
At least ten days before the experiments, anthropometric and 1-repetition maximum (1RM) measurements were collected, and the subjects were familiarized with the exercise scheme. The ratio of total muscle strength (the sum of six different resistance exercises, consisting of supine bench press, sitting shoulder press, leg press, hamstring curl, two-arm biceps curl, and lat pull-down) to body mass (kg) was used to measure relative muscle strength.
3.5. Supplement and Dietary Conditions
The subjects were provided either gelatin capsules containing strawberry extract or an identical powdered maltodextrin placebo containing 250 mL of water. Two capsules (each containing 350mg) were taken after lunch and dinner each day for 14 days. Four weeks washout period was applied, then the groups were replaced. The subjects confirmed the use of the supplement by sending a text message to the researcher during the 14 days of supplementation.
All subjects were asked to avoid vigorous physical activity and maintain normal dietary patterns and consumption of caffeine or alcohol in the 72h before the resistance exercise test. Table 2 presents an average of the values for food intake during the study. The subjects completed diet records, beginning on the week before the study and during the study (using a 7-day food record). The Singleton and Rossi method determined the total polyphenol contents in food products (19). Dietary intakes included all three provided energy (carbohydrates, proteins, fats; measured in calories) and vitamins A, C, and E (as an antioxidant), which were evaluated by the Nutritionist IV software (The Hearst Corporation Bayhill Dr, San Bruno, CA 94066). The body fat percentage was estimated using a bioelectrical impedance analyzer (BIA; TBF-770, South Korea).
| Variables | Strawberry Extract, Mean ± SD | Placebo, Mean ± SD | P-Value |
|---|---|---|---|
| Energy intake, kcal | 2102.8 ± 402.9 | 2264.3 ± 553.4 | 0.184 |
| Protein, g | 61.4 ± 24.6 | 64.2 ± 43.7 | 0.255 |
| Carbohydrate, g | 375.2 ± 87.8 | 401.6 ± 136.1 | 0.341 |
| Fat, g | 39.6 ± 28.9 | 45.3 ± 32.4 | 0.182 |
| Vitamin A, RE | 41.5 ± 11.2 | 36.5 ± 11.4 | 0.354 |
| Vitamin C, mg | 275.4 ± 98.1 | 215.7 ± 96.8 | 0.551 |
| Vitamin E, mg | 36.5 ± 15.3 | 41.6 ± 23.5 | 0.367 |
| Mean polyphenol intake, mg/person/day | 1793.5 ± 395.8 | 963.3 ± 436.7 | 0.001 a |
a A significant difference between strawberry extract and placebo groups (P < 0.05)
3.6. Exercise Protocol
Women repeated the same bout resistance exercise test after two weeks of supplementation (on day 15). The protocol began with a short warm-up. The subjects then completed four sets to failure repetitions of six different resistance exercises at 80% of 1RM with an interval of 60s between sets and 120s between the exercises. All subjects received verbal encouragement during exercise. This protocol has already been shown to significantly increase serum apoptosis biomarkers (3, 20).
3.7. Ethical Approval
This study was approved by the Institutional Review Board of the University of Kurdistan (IR.UOK.REC.1399.007) and completed based on the standards set by the latest revision of the Declaration of Helsinki. All subjects gave their written consent before their inclusion in the study.
3.8. Blood Sampling
The blood samples from the antecubital vein (10 mL) were obtained by Vacutainer pre-supplementation (after 12 h fasting) and after two weeks (it was repeated). Blood samples were collected immediately following resistance exercise on the 15th day, as a potential decrease in serum apoptosis marker concentrations post-exercise (8). The serum samples were placed at room temperature for 20 min after being transferred to the tubes. The serum was obtained after 20 min of centrifugation (3,000 rpm, 4°C) and stored at -20°C until further analysis. The cytochrome c and caspase-3 concentrations were measured for ELISA by special kits (Human ELISA, Bioassay Technology Laboratory, Shanghai Crystal Day Biotech Co., Ltd. China). The detection limit of this method was 0.09 ng/mL. The intra- and inter-assay CV were less than 10% for blood cytochrome c and caspase-3.
3.9. Statistical Analysis
The statistical analysis was performed by SPSS software Version 22.0 in Windows. Normal distribution for all variables was verified with the Shapiro-Wilk test. The independent-sample t-test was used to compare the means of values in Tables 1 and 2. The effect sizes as small (d = 0.2), medium (d = 0.5), and extensive (d = 0.8) were based on benchmarks suggested by Cohen (21). Cohen indicated that d = 0.2 be considered a ‘small’ effect size, 0.5 represents a ‘medium’ effect size, and 0.8 a ‘large’ effect size. η2 measures effect size and reflects the percentage of the variance in the dependent variable explained by the independent variables in a sample. All parameters were compared using two groups (supplemented vs. placebo) × 3 times (pre-supplementation vs. post-supplementation vs. post-exercise), repeated measures analysis of variance (ANOVA), and post hoc Bonferroni test. For all repeated-measures ANOVAs, Mauchly’s test of sphericity was performed to assess the equality of variances between groups. All the data were expressed as means ± SD, and the statistical significance was accepted for P < 0.05.
4. Results
All subjects completed the acute resistance exercise session. The data had a normal distribution, and the assumption of homogeneity of variance was violated. No differences were observed in resting levels of cytochrome c and caspase-3 between PL and SES groups (P > 0.05; Figures 1 and 2). No significant differences were observed in cytochrome c and caspase-3 levels before and after a two-week supplementation in both groups of PL and SES (P > 0.05).
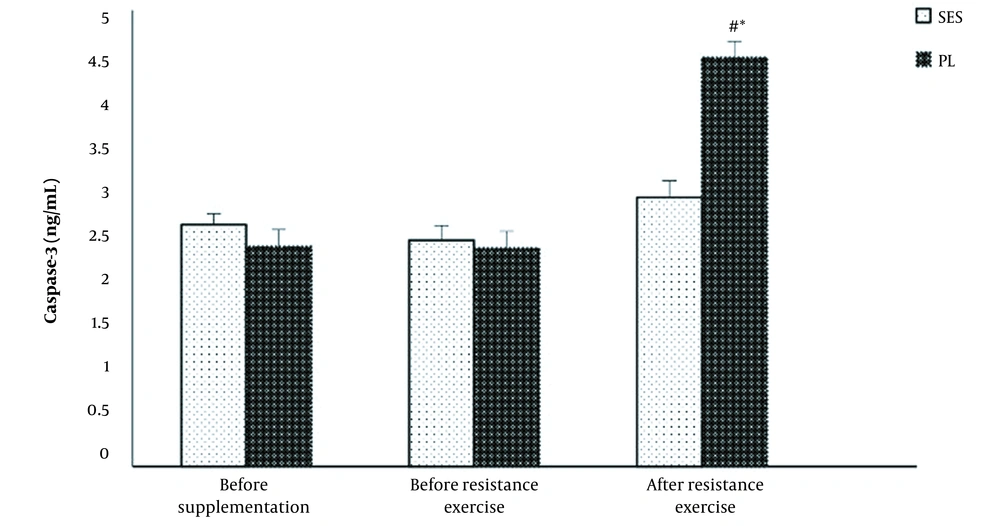
Figure 1 illustrates responses of caspase-3 levels to acute resistance exercise after PL or SES supplementation. Significant time effects (F = 66.42, P = 0.001, η2 = 0.82), group (F = 19.47, P = 0.001, η2 = 0.58) and interaction (F = 46.46, P = 0.001, η2 = 0.76) were found for caspase-3 levels. Serum caspase-3 increased in the PL group (2.39 ± 0.3 vs. 4.48 ± 0.4 ng/mL by 87.4 ± 8.3%; P = 0.001) after acute resistance exercise protocol compared to pre-resistance exercise. However, changes in caspase-3 did not reach statistical significance for the SES group (2.48 ± 0.4 vs. 2.95 ± 0.5 ng/mL by 18.9 ± 7.8%; P = 0.149).
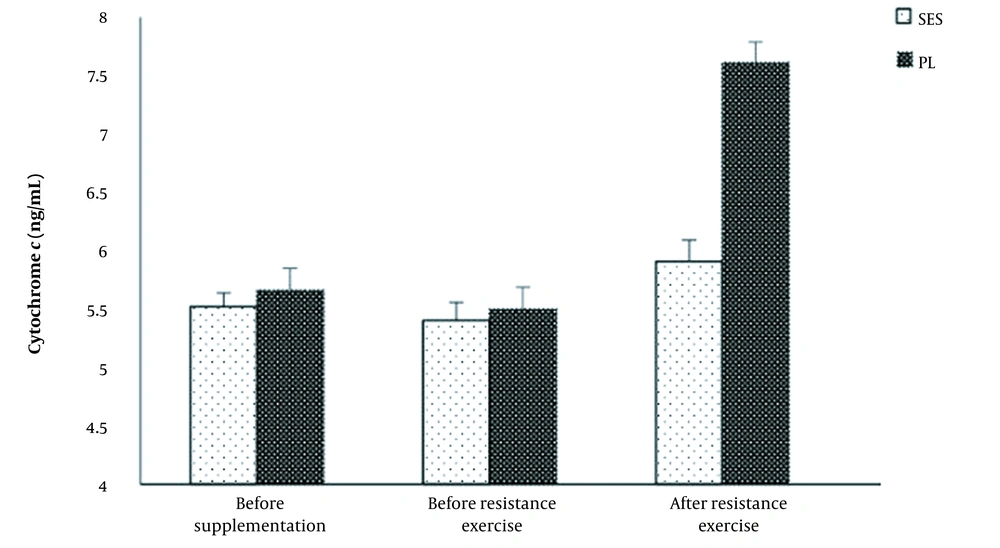
There were a significant time effects (F = 103.38, P = 0.001, η2 = 0.88), group (F = 79.46, P = 0.001, η2 = 0.85) and interaction (F = 76.97, P = 0.001, η2 = 0.84) for cytochrome c levels. Cytochrome c was significantly higher after acute resistance exercise protocol (5.50 ± 0.2 vs. 7.66 ± 0.1 ng/mL by 39.2 ± 4.5%; P = 0.001), compared to pre-resistance exercise (Figure 2). In the SES group, cytochrome c concentrations were slightly higher (but not significantly) compared to pre-resistance exercise (5.40 ± 0.2 vs. 5.91 ± 0.3 ng/mL by 9.4 ± 3.7%; P = 0.108).
A significant difference was observed between the PL and the SES in caspase-3 (P = 0.001) and cytochrome c (P = 0.001) concentrations immediately after the post-resistance exercise protocol (Figures 1 and 2).
Changes in serum caspase-3 levels at pre- to post-supplementation and after the acute resistance exercise protocol. Values are means ± SD. The P-values indicate the results of a Bonferroni post-hoc analysis. # Significant difference between strawberry extract (SES) and placebo (PL) groups (P < 0.05). * Significantly different from pre-resistance exercise values (P < 0.05)
Changes in serum cytochrome c levels at pre- to post-supplementation and after the acute resistance exercise protocol. Values are mean ± SD. The P-values indicate the results of a Bonferroni post-hoc analysis. # Significant difference between strawberry extract (SES) and placebo (PL) groups (P < 0.05). * Significantly different from pre-resistance exercise values (P < 0.05)
5. Discussion
The intense resistance exercise increased serum apoptosis biomarkers in healthy women. Strawberry extract modulated exercise-induced caspase-3 and cytochrome c levels.
Apoptosis is considered a vital component of various processes including normal cell turnover, proper development and functioning of the immune system. However, inappropriate apoptosis (either too little or too much) had a negative role in maintaining the function, wasting, atrophy, or sarcopenia of skeletal muscle, which could yield tissue degeneration (i.e., lymphocytes, heart) (22, 23). There may be a basis for this phenomenon in the concept of hormesis, which can be described as a dose-response relationship in which a low dose is stimulatory and a high dose is inhibitory.
The strawberry extract had higher natural antioxidant effects than plums, tomatoes, red grapes, oranges, and bananas (24). Strawberry is a good source of extra nutritional constituents (such as carotenoids, polyphenols, and glucosinolates), vitamins, and minerals. These compounds can effectively reduce ROS, DNA damage, and inflammatory markers (11). The strawberry phenolics could stabilize free radicals (including superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen) and regulates the expression of genes involved in cell growth and proliferation (11, 25). Etemad and Rasouli showed that 2 wk strawberry extract supplementation attenuated fibrinogen concentrations, as inflammatory markers, following endurance exercise within 75 - 80% of the maximum heart rate in inactive women (18). Basu et al. observed a significant increase in antioxidant biomarkers in obese men receiving dietary freeze-dried strawberries supplementation (12).
To the best of our knowledge, this is the first research describing the effect of two-week strawberry extract intake on circulation apoptosis markers after resistance exercise in nonathletic subjects. Administration of strawberry attenuated ROS and inflammatory factors (12, 15). Therefore, it was expected that strawberry extract could attenuate cell death pathways, which modify exercise-induced apoptosis. For this purpose, cytochrome c and caspase-3 were measured in two groups of women: one group consumed a supplement (strawberry extract), while the other group received a placebo (maltodextrin). The research demonstrated that a single bout of eccentric exercise evoked apoptosis markers, and intake of strawberry extract supplements could reverse exercise-induced apoptosis in women.
An eccentric contraction of resistance exercise induces cell damage, increases ROS and inflammatory markers, and alters immune parameters and cell survival (8, 26). Some previously reviewed studies’ results provided evidence that an increase in apoptotic markers marks resistance to exercise-induced apoptosis. Results from a study reported that caspase-9 and p53 protein were increased following high-intensity resistance exercise in untrained individuals (2, 20). Furthermore, resistance exercise increased blood caspase-3 levels after one bout of resistance exercise in nonathletic. A single bout of intense exercise increased muscle caspase-3 (27) and Bax concentrations (28). The underlying mechanism(s) of eccentric exercise-induced apoptosis was not fully clarified. However, it has been proposed that ROS has an essential role (8). Excessive ROS can limit cell life by activating the death receptor signal or disrupting the regulation of the mitochondrial membrane potential (7). Nevertheless, the acute resistance exercise at 80% 1RM was a sufficient stimulus to evoke exercise induced-apoptosis via increased cytochrome c and caspase-3 in untrained women.
The release of cytochrome c from mitochondria is a key initial step in the apoptotic process (4). Serum cytochrome c has been measured as an apoptosis marker (10). The current study demonstrated that cytochrome c, a circulatory marker, increased after resistance exercise in the PL subjects. Sheikholeslami-Vatani et al. reported that high-intensity resistance exercise (80% of 1RM) increased cytochrome c in older adults (8). Moreover, acute intense exercise increased cytosolic cytochrome c levels (29).
There were no significant changes in cytochrome c response in the SES subjects. Therefore, the strawberry extract prevented mitochondrial stress by preventing cytochrome c release. The possible mechanism(s) of strawberry on cytochrome c level remains unclear. As mentioned earlier, mitochondrial structures are exposed to high concentrations of oxidative injury and may be particularly susceptible to their attack. Cytochrome c is released from the intermembrane space of the mitochondria into the cytoplasm in response to excess apoptotic stimuli (i.e., ROS, TNF-α, Bax, FasL, and caspases-2), subsequently leading to apoptotic cell death (30). A possible mechanism(s) for these results may be related to the exercise-induced apoptotic stimuli. Based on a study, that pretreatment with strawberry phenolics suppressed PC12 cell apoptosis by oxidative stress (24). ROS could directly induce the segregation of cytochrome c from the inner mitochondrial membrane and subsequently release it from the organelle (5). The ROS production was decreased by strawberry extract in myometrial normal cells.
In contrast, ROS production and the percentage of apoptotic cells were significantly higher in strawberry-treated of leiomyoma cells (31). On the other hand, strawberry was accompanied by a decrease in TNF-α and free radicals (32). However, strawberry extract, rich in anthocyanins and polyphenols, protected cells from cell death by blocking caspase-9 and -3 activation, restraining mitochondrial membrane potential dissipation, and preventing intracellular ROS as elevating cytoplasmatic Bax levels and inactivating the PI3 K/Akt pathway (32).
Theoretically, caspase-3 is a hallmark of apoptosis and the terminal event preceding cell death (33). The caspase-3 multiple pathways (by the upstream caspase-8, 9, or 12) play a role in triggering the caspase-3 activation. The results showed that the strawberry extract prevented the exercise-induced increase of serum caspase-3 in healthy women compared to the control session. The findings of elevated caspase-3 after one bout of extreme exercise supported previous research. Circulating levels of caspase-3 elevated following intense resistance exercise in untrained men (3). One bout of strenuous treadmill running (12% incline) at 70% of HRmax for 40min increased serum caspase-3 levels in middle-aged men (34). Moderate-intensity resistance exercise (65% 1RM) increased caspase-3 mRNA in untrained subjects (35). Nevertheless, the caspase-3 levels were not significantly altered (slight increase) in the SES trial. No study to date has investigated the effects of strawberries on caspse-3 with or without acute exercise. Only one study reported a significant protective effect of the methanolic strawberry extract by activating the intrinsic pathway of apoptosis (activating p73, p53, caspase-3, and caspase-9) to induce cell death in cancer cells (36). However, this study did not assess their effect on normal cells. The current data proved that the strawberry extract could favor caspase-3 (relative to placebo) by attenuating cytochrome c. In addition, some studies have shown attenuated effects of strawberries on intrinsic or extrinsic pathways of apoptosis initiators and increased IL-10 (an anti-inflammatory cytokine) (37) and Bcl-2 (anti-proapoptotic protein) (16). Although more study is needed, the results suggest that cell protection by strawberries is partially due to the mitochondrial protection mechanisms (32).
The current study has some advantages, but there are no limitations. The sample size is relatively small and includes healthy young untrained women. Therefore, the results cannot be generalized to unhealthy, older, or trained adults, and the results might not represent the metabolic/hormonal changes in men. In this study, markers of oxidative stress were not determined. Only one intrinsic pathway factor was considered to better measure extrinsic pathway-related factors of cell death.
Furthermore, tissue biopsies were not obtained, but the effects of cell apoptosis can be detected by measuring the circulation by measuring serum cytochrome c (as apoptosis initiator) and caspase-3 (as effector caspase) (38). Based on previous studies (39), mixtures of ethanol/water were more efficient in extracting phenolic compounds. Considering that the goal was not to separate the contents of the extract and the overall effect of the extract with the placebo on the research variables were only compared, solvents were not used. This case was another limitation of our research. Today’s method of measuring total phenol is based on the Folin–Ciocalteu method; however, we used another technique for extraction.
Similar to topics related to oxidative stress and exercise-induced inflammation, the optimal amount of apoptosis is not precise due to strenuous acute activity for cellular adaptation. However, uncontrolled cell death can lead to decreased exercise performance and optimal muscle adaptation. Elevating the intracellular antioxidants should be protected against these oxidizing agents and reduce fatigue (40).
5.1. Conclusions
In conclusion, 14 days of strawberry extract supplementation inhibited increased cytochrome c (as apoptosis initiator) and caspase-3 (as the execution of apoptosis) induced by resistance exercise in young women. Although the mechanism by which the strawberry extract exerts its cellular protection effect against cellular apoptosis was not understood at the molecular level, the cell protection by strawberries was partially due to the mitochondrial protection mechanisms. These novel findings suggested the ability of the strawberry extract to maintain homeostatic conditions in normal cells.