1. Background
Transdermal delivery is based on the permeation of drugs through the skin (1). Avoidance of first-pass metabolism, regulated and continuous drug administration, lower dosage frequency, improved patient compliance, drug delivery at the target location, and reduced side effects and toxicity are some of the benefits of transdermal drug delivery (2-8). Vehicle substances like surfactants and solvents are used for drug delivery for different skin layers, which can alter the permeability- properties of the skin (9). This permeation is dependent on physicochemical properties, as well as solubility and partition coefficient. An appropriate solvent mixture may significantly affect drug delivery from topical preparations in this type of drug delivery. Propylene glycol, water, ethanol, transcutol P, caprylic acid, and isopropyl alcohol are some of the solvents that can increase drug permeation through a number of different mechanisms, including the disruption of intercellular lipid structures, fluidization of stratum corneum (SC) lipids, modification of cellular proteins, and extraction of intercellular lipids. Polar solvents, such as dimethyl sulfoxide (DMSO) and dimethyl form amide, were shown to enhance the stratum corneum's diffusely (DMFA) (10, 11). Minoxidil is the most common drug used to treat hair loss, which is available as a generic medication over-the-counter for treating androgenic alopecia and, a form of hair loss in people with a molecular weight of 209.253 g/mol. The effectiveness of the absorption from Eucalyptus oil, Menthol, and Urea as the main likely mechanisms for higher ERflux, ERD, and ERP ratios compared with water-hydrated skin was shown by minoxidil percutaneous absorption in the rat. This was true regardless of the amount of minoxidil applied to a specific area (12).
2. Objectives
The goal of this research was to create and develop an appropriate transdermal drug delivery system for minoxidil by examining the impact of different vehicles on the in vitro skin permeability of minoxidil.
3. Methods
Sepiddaru Company (Tehran, Iran) supplied the minoxidil, while Merck supplied the propylene glycol, ethanol, isopropyl alcohol, caprylic acid, and transcutol P (German). Analytical grades were given for all other materials.
Animals laboratory at Jundishapur University of Medical Sciences in Ahvaz, Iran, sold male adult Wistar rats weighing 100 - 150 g that were 10 - 12 weeks old. Without causing damage, the hair was removed from the abdomen skin using a clipper. Before being sacrificed, ether was used to make the rats unconscious. The dorsal side of the abdomen was cleansed of superfluous subcutaneous fats using a cooled pure acetone solution at 4°C after the complete thickness of the abdominal skin was removed. Digital micrometer was used to measure the thickness of the whole skin layer (AAOC, France). The Ahvaz Jundishapur University of Medical Sciences' Ethical Committee authorized animal experiments in accordance with the guidelines for the handling and use of laboratory animals. The steps taken were in accordance with the moral code and generally accepted international standards (IR.AJUMS.ABHC.REC.1397.19).
Using a DSC (Mettler Toledo DSC1 system) outfitted with a refrigerated chilling system, the changes in the overall structure of the skin brought on by solvents were investigated (Hubert Tc45). Aluminum pans with hermetically sealed lids were filled with 5 - 10 mg of the sample, while an empty hermetically sealed pan served as a control. Skin samples were heated to temperatures between 20 and 200°C at a scan rate of 5°C/min. All tests were performed at least three times. To assure accuracy and repeatability, the DSC analyzer was calibrated and verified using an indium reference. Equation 1 was used to get the values of enthalpy (ΔH) from the thermograms' endothermic and exothermic transitions.
Extracted rat skin samples were treated with transcutol, caprylic acid, propylene glycol, water, isopropyl alcohol, ethanol for 4 h, vacuum-dried (650 mmHg, 25 ± 1°C) for 1 h, and kept in desiccators to remove solvent. FT-IR scanned the samples between 4000 and 500 cm-1.
In-vitro permeation studies were performed using vertical glass Franz diffusion cells with an approximated effective diffusion area of 5.73 cm2. Receptor chamber held 30 mL. The complete skin sample was moistened and mounted with the stratum corneum facing the donor media. Minoxidil (1% w/v) in test solvent was in donor compartment; phosphate buffer was in receptor cell (pH: 7). Franz diffusion cells were stirred in 370.5°C water. Magnets stirred the receptor medium. At 0.5, 1, 2, 3, 4, 5, 6, 7, 8 and 24 h, 2 mL of receptor medium was removed and replaced with new phosphate buffer (pH: 7). UV spectroscopy at 290 nm was used to measure indomethacin permeation in filtered samples. Each test solvent was utilized as a blank to measure flux (Jss), permeability coefficient (P), lag time (Tlag), and diffusivity coefficient (D). The steady-state permeation rate (Jss, mg/cm2.h) was determined from the linear slope of the permeation curve. Extrapolating the steady-state line to the time axis yielded Tlag (hr) (13).
The cumulative permeated minoxidil per unit area was calculated through dividing the corrected minoxidil concentration by the area of the skin exposed to the donor solution. The steady-state flux (mg/cm2.h) was calculated from the linear portion of the slope of the permeation curve. The permeability coefficient (Kp, cm/h) through the skin for minoxidil was determined as in Equation 2. Minoxidil concentration was corrected for sampling effects according to Equation 2.
where Jss and Cv are steady-state flux and minoxidil concentration in the donor phase, respectively.
All studies were done in triplicate, and findings were given as mean ± SD using one-way ANOVA. Statistically, P-values less than 0.05 were significant (13).
4. Results and Discussion
Table 1 shows the solubility of minoxidil in different vehicles. The results indicated the highest solubility in ethanol, followed by PG, water, transcutol, caprylic acid, isopropyl alcohol, and propylene glycol.
| Vehicles | Solubility (mg/mL) |
|---|---|
| Ethanol | 28.2 ± 1.2 |
| PG | 73 ± 0.01 |
| Water | 2.3 ± 0.1 |
| Transcutol | 4.5 ± 0.5 |
| Caprylic acid | 14.5 ± 1.5 |
| Isopropyl alcohol | 6.1 ± 0.6 |
Table 2 shows the influence of vehicles on minoxidil skin permeability (hydrated skin as control) (drug diffusion coefficient skin pretreatment). PG, ethanol, and water had the least influence on drug permeability. Due to rising diffusion, PG and water had a higher ERflux than ERD.
| Enhancer | Jss (mg/cm2.h) | Dapp (cm2/h) | P (cm/h) | Tlag (h) | ERflux | ERD | ERp |
|---|---|---|---|---|---|---|---|
| Control | - | - | - | - | - | - | - |
| Water | 0.1128 ± 0.0561 | 0.127 ± 0.1852 | 0.0056 ± 0.0028 | 1.696 ± 1.343 | - | - | - |
| Ethanol | 0.0434 ± 0.0013 | 0.0044 ± 0.011 | 0.0021 ± 6.71E-5 | 1.178 ± 0.314 | 4.721 ± 0.145 | 12.381 ± 3.064 | 2.360 ± 0.072 |
| Isopropyl alcohol | 0.035 ± 0.004 | 0.1138 ± 0.1348 | 0.00146 ± 0.001 | 1.16 ± 1.069 | 3.880 ± 0.439 | 31.515 ± 37.325 | 1.940 ± 0.219 |
| Transcutol | 0.029 ± 0.0228 | 0.0479 ± 0.065 | 0.0014 ± 0.0011 | 3.747 ± 3.29 | 3.188 ± 2.48 | 13.271 ± 18.132 | 1.594 ± 1.24 |
| Caprylic acid | 0.1627 ± 0.0624 | 0.024 ± 0.0142 | 0.0008 ± 0.0031 | 2.47 ± 1.243 | 17.688 ± 6.783 | 6.904 ± 3.943 | 8.845 ± 3.392 |
| PG | 0.0323 ± 0.323 | 0.0725 ± 0.056 | 0.0019 ± 0.0016 | 0.985 ± 0.583 | 4.336 ± 3.51 | 20.095 ± 15.651 | 2.168 ± 1.757 |
Drug flux through rat skin was decreased more by all vehicles than by diffusion. The high solubility of minoxidil in ethanol suggests that ethanol is an appropriate carrier for drug solubility stratum corneum. Table 1 shows the solubility of minoxidil in various vehicles. The results indicated the highest solubility in ethanol, followed by PG, water, transcutol, caprylic acid and isopropyl alcohol (11).
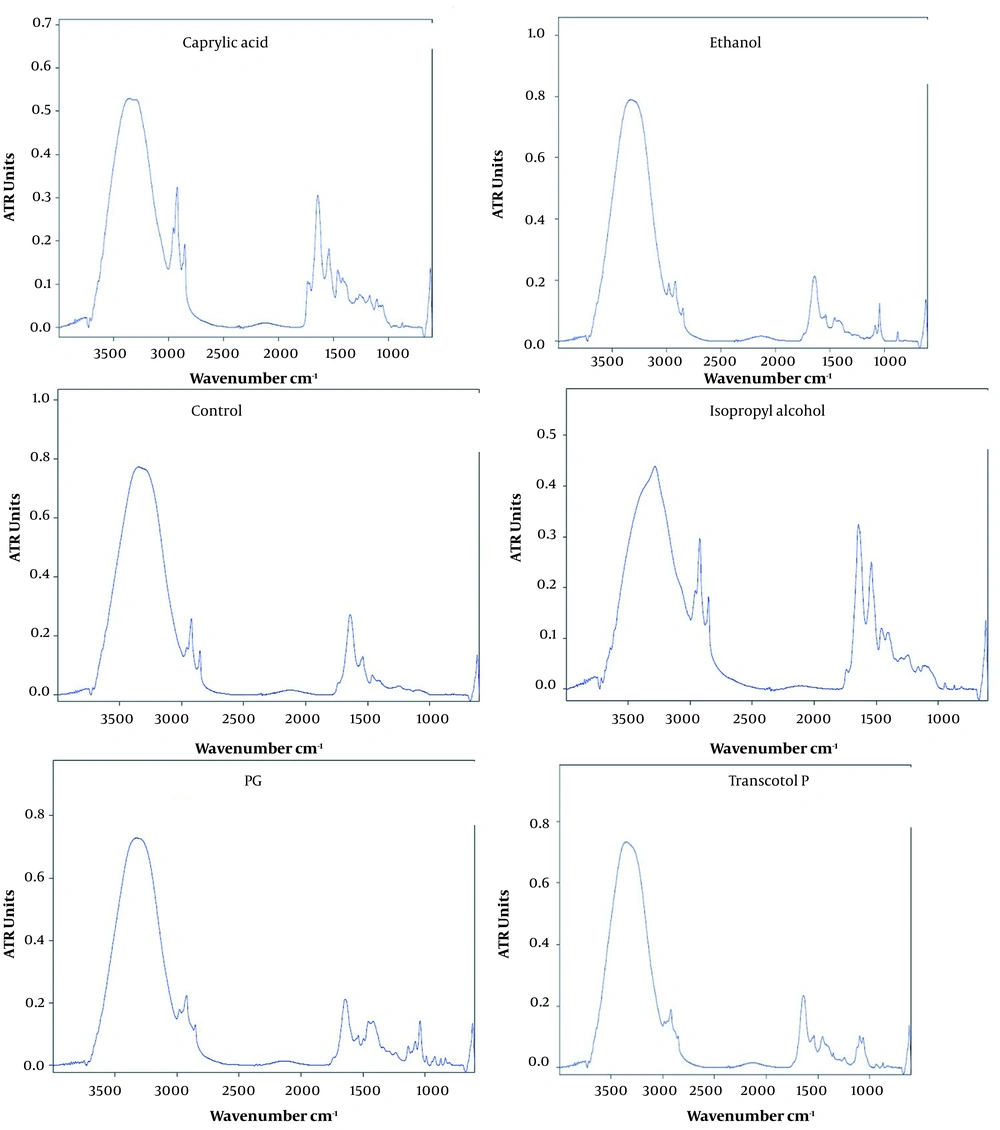
The spectral analysis examined the changes in peak position and their intensities from 500 - 4200 cm-1 (Figure 1 and Table 3). In this study, untreated whole rat skin served as the control and showed bands at 500 - 4200 cm-1. The bands observed in the range of 3000 - 3600 cm-1 represented O-H and N-H stretching from lipid, protein, and water. However, the peaks near 2919.59, 2850.058, 1642.89, 1540.26, 1463.71, 1246.83, 1102.55, 618.17 cm-1 presented asymmetric and symmetric stretching bands of the terminal methyl groups of lipids in rat skin. The lipid ester carbonyl stretching in SC showed at 1723.62 cm-1 of position, while the bands observed at 1652.78 and 1572.27 cm-1 represented amide I (C=O stretching) and amide II (C-N stretching) linkage of the helical secondary structure found in epidermal keratin (14).
| Enhancer | Amide1 Stretching of Keratin | Amide2 Stretching of Keratin | C-H Stretching Asy | C-H Stretching Sym | C=O Stretching of Lipid Ester | Amide І | Amide ІІ | ||
|---|---|---|---|---|---|---|---|---|---|
| Wave Number | %D | Wave Number | %D | ||||||
| Water | 0.260 | - | 0.13 | - | 2921.215 | 2851.673 | 1743.412 | 1634.552 | 1552.566 |
| Isopropyl alcohol | 0.324 | Not seen | 0.251 | Not seen | 2929.974 | 2851.362 | - | 1644.660 | 1457.908 |
| Transcutol | 0.234 | 10 | 0.105 | 19.230 | 2983.099 | 2919.170 | - | 1643.256 | 1540.152 |
| Ethanol | 0.212 | 18.461 | 0.880 | Not seen | 2981.247 | 2920.273 | - | 1643.618 ± 0.1 | 1539.670 ± 0.3 |
| Caprylic acid | 0.306 | Not seen | 0.182 | 3 | - | 2919.553 | - | 1643.813 ± 0.2 | 1540.178 |
| PG | 0.96 | Not seen | 0.141 | 3 | 2921.2 | 2850.825 | 1645.837 | 1539.592 | 1456.915 |
An FTIR spectrum obtained from the SC shows caprylic acid eliminates the peak in the asymmetric traction absorption band and increases the absorption band significantly. This solvent also increased the wavelength in the amide area, and the caprylic acid affected the lipid section and protein content of the skin. Furthermore, the percentage reduction in lipids in the protein in this solvent is justified, suggesting a significant effect on skin lipids. The ethanol increased in the symmetric and asymmetric region of CH, indicating that the alcohol causes the absorption bands in these regions to move toward wavelengths high. Alcohol led to fluid bilayers and increased the passage of drugs through the skin. Transcutol was significantly reduced, and the peak height was absorbed in the regions. This solvent removed the peak in the regions, and this carrier created a water shift similar to symmetric and non-symmetric CH. The results showed that transcutol increased the minoxidil passage due to disorder in the lipid region of the SC tissue and disruption of the SC layer. The carrier’s decrease in courier height in the amid region indicates applying this compound through the horny protein sector. The isopropyl alcohol removed the peak in the absorbing region with no significant change in absorption bands. In addition, this solvent significantly reduced the peak height in symmetric and asymmetric CH regions of amide I, II. The carbonyl groups were stretched, indicating irregularities in the SC through the effect of lipid and proteins in the skin and ultimately increases the passage of drug from the skin. Propylene glycol solvent was not considerably displaced in the asymmetric and symmetric traction band of the end methyl groups of the lipid structure in rats' skin, reducing the absorption band's bandwidth. All instances increase SC barrier characteristics. This solvent lowered gradients in desirable locations. Since the height or area of the links reflects the amount of protein lipids within the SC, reducing the courier height increases the intensity or height of a bond, particularly rendering. Therefore, propylene glycol decreases the intensity and height of the courtyard in the SC lipid and proteins.
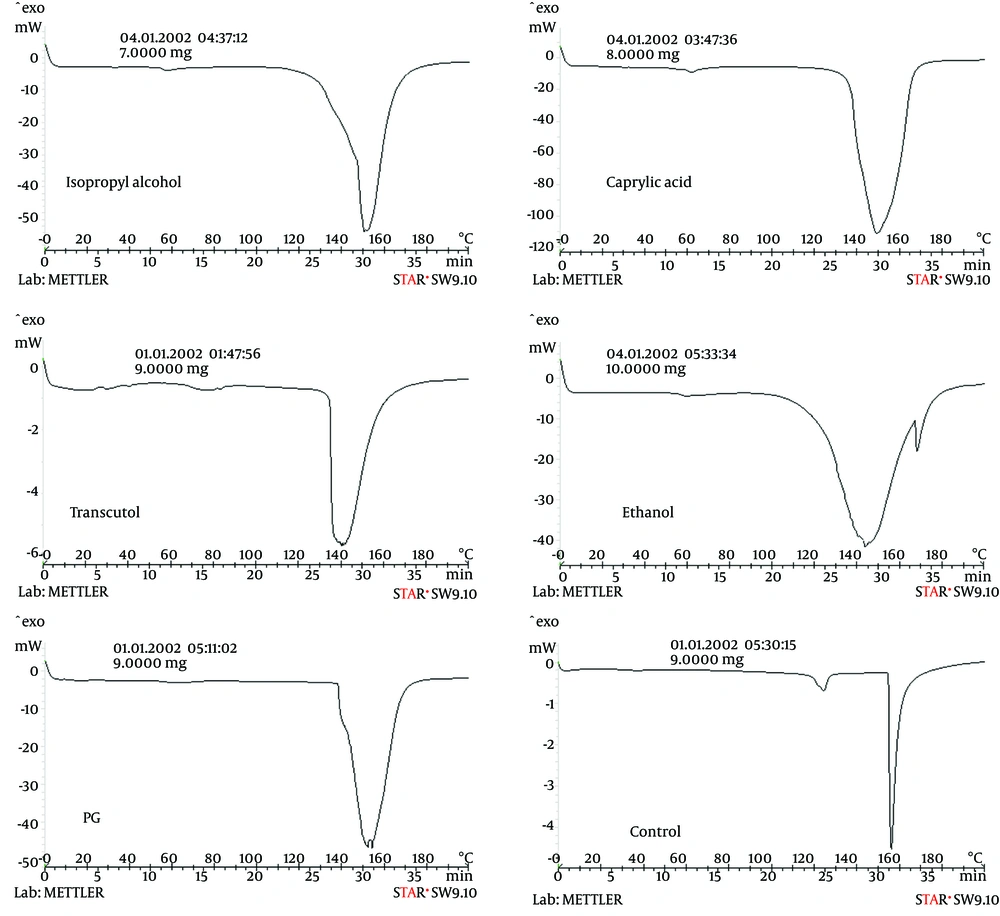
The thermotropic behavior of skin treated with various vehicles was evaluated by comparing mean transition temperature (Tm) and their enthalpies (ΔH).
In this study, two endothermic transitions were obtained at around 30 - 60°C (T1) and 100 - 160°C (T2) in a thermogram of hydrated whole rat skin. There was irreversible denaturation of intracellular keratin during the T1 and T2 transitions, respectively. Table 4 shows that decreasing T1 and T2 and increasing ΔH1 and ΔH2 shifted to higher temperatures by all vehicles showing their interaction with the lipid layer and possible denaturation of skin protein.
| Enhancer | Penetration Enhancer | Transition Temperature (°C) | Transition Enthalpy (mj\mg) | |
|---|---|---|---|---|
| Tm1 | Tm2 | ΔH1 | ΔH2 | |
| Water (control) | 67.5 ± 2.1 | 112 ± 6.6 | -7.01 ± 0.4 | -551.25 ± 19.5 |
| Isopropyl alcohol | 56 | 152 | 6.077 | 1480.8 ± 0.3 |
| Transcutol | 26 | 75 | 0.498 | 100.74 |
| Ethanol | 61 | 144 | 3.71 | 1030.29 ± 0.3 |
| Caprylic acid | 64 | 150 | 12.17 | 3090.27 ± 0.3 |
| PG | 59 | 153 | 4.13 | 1010 |
FTIR and DSC results showed that all carriers increased the drug's permeability from the rat's skin compared to the control. Caprylic acid has the most effect on flux and then on ethanol propylene and glycol. Isopropyl alcohol has the highest ERD of the drug from the rats’ skin, followed by propylene glycol (Figure 2).
The minoxidil molecule weight is 252.89 Dalton, and its logp is 1.02. The compound can accept three hydrogen bonds and create three hydrogen bonds. The flux contains the partitioning and diffusion processes. Therefore, all vehicles increase the donor of the drug into the skin than the diffusion. Minoxidil is a low-solubility drug in water, and its solubility in the stratum corneum of the skin determines its distribution. Therefore, all carriers are intended to increase the drug solubility in the stratum corneum and enhance the flux and distribution. The highest solubility of minoxidil is in PG (73 ± 0.01), followed by ethanol (28.2 ± 1.2) and capriol (14.5 ± 1.5), isopropyl alcohol (6.1 ± 0.6), and transcutol (4.5 ± 0.5) and water (2.3 ± 0.1). After extended skin contact with the propylene glycol carrier, the FTIR spectrum shows no substantial displacement of the asymmetric and symmetric traction bands of the end methyl groups of the lipid structure in rats' skin, and the carrier diminishes the absorption band's bandwidth. This strengthens the stratum corneum barrier.
Nevertheless, this carrier significantly reduced the gradient in the desired areas. Since the height or area of the bonds show the number of protein lipids inside the stratum corneum, decreasing the height of the courier increases the intensity or height of a bond, especially rendering. Therefore, propylene glycol decreased the intensity and height of the courtyard in the lipid and proteins of the stratum corneum. DSC results from the isopropyl solvent effect decreased Tm1 and ΔH2 compared to the control. As a result, the lipid layer fluidizes, lipid-protein complexes become disordered, and the protein structure is irreversibly altered. Caprylic acid reduced Tm1 and declined ΔH1 and ΔH2. In the DSC, fluidization of the lipid layer, disorder of the lipid layer, and denaturation of the protein structure are consistent with FT-IR data. The effect of transcotole solvent indicates the increased Tm2, ΔH, and the ethanol reduced Tm1, ΔH1 and ΔH2. As a result, olive oil disrupts the lipid layer biliary fluidization, and lipid-protein complexes are present in SCs. The effect of water solvent on the skin indicates that water reduced Tm1 and Tm2 compared to control, showing the effect of this compound on the lipid-protein complex in SC. This compound reduced ΔH1 and ΔH2, indicating the lipid fluidity in the Bylier and lipid-protein complex of the SC layer.
Solubility in stratum corneum and drug-skin hydrogen bonding determine drug partitioning. FT-IR and DSC data show that PG interacts predominantly with SC keratins and does not change SC lipid structure. The change to a lower wave number reoriented lipid groups, increased SC barrier characteristics, and slowed minoxidil transcutol transit. 1000 - 2000 cm-1 shows hydrogen bonding in lipid molecules. As expected, these vehicles penetrate SC membrane lipids (15). Water, transcutol, ethanol, and PG all have lower ERD ratios due to lipid fluidization and extraction by isopropyl alcohol and caprylic acid, as indicated by FT-IR. Caprylic acid increases enthalpy and transition Tm1 because of bilayer cohesion (16), as opposed to lipid extraction and fluidization. Increasing Tm caused disruption of the lipid bilayer and denaturation of the protein in the SC layer, and lowering ∆H caused fluidization of the lipid bilayer and lipid-protein complex (14, 17-21). The melting point of stratum corneum lipids is decreased by transcutol, which is oil-induced. As a consequence of oily induction, capriol causes a rise in the melting point of lipids found in the stratum corneum. Caprylic acid largely interacts with SC keratins in rat skin, which results in alterations to the structures of lipids.
5. Discussion
5.1. Conclusions
According to the results, all the tested vehicles increased permeability across rat skin. The main probable mechanisms for the higher ERD ratio were lipid fluidization, disruption of lipid structure, and irreversible denaturation of keratin within the stratum corneum by isopropyl.