1. Background
International classification of sleep disorders (ICSD), second edition defines insomnia disorder as difficulty in starting, maintaining, or stabilizing sleep when there is ample opportunity (1, 2). The patient usually reports daily disturbances associated with nocturnal sleep problems, such as fatigue, difficulty concentrating, impaired social and work functioning, unstable mood, daytime sleepiness, decreased motivation, physical symptoms, or excessive worry about sleep (3-5). There are some clinical and pathological subtypes of ID, including psychophysiological insomnia, idiopathic insomnia, and paradoxical insomnia (Par-I) (5). The symptoms of the Par-I condition include subjective complaints of severe insomnia, which do not correlate with objective sleep measures, such as polysomnography (1, 3, 5, 6). Par-I patients usually underestimate their total sleep time, the period between sleep onset and waking, and the period between going to bed and sleeping (7, 8).
Recently, Krystal et al. compared the power of brain signals in different frequency bands and observed significant differences in alpha, beta, theta, and gamma bands between patients with Par-I and healthy individuals (9). Moreover, Harvey and Tang (10) hypothesized three possible mechanisms to potentially explain the discrepancy between subjective reports and objective measures as follows: (1) short-term awakenings during the night and early morning; (2) worry and selective attention to sleep-related concerns; and (3) misunderstand sleep as wakefulness. The misperception of sleep in Par-I may also be caused by the presence of local wakefulness and sleep (11, 12).
According to research on Par-I, local processes can have an essential role in the misperception of the sleep state. The purpose of this study was to evaluate possible differences in ALFF as a reliable marker of local processing (13), obtained by resting-state fMRI among 15 people with Par-I and 48 healthy people (HC).
2. Objectives
This study seeks to check whether two Par-I and HC groups significantly differ in the extracted ALFF.
3. Methods
A total of 79 participants were included in this study during 2018 - 2019. Patients with ID were selected from the Sleep Disorders Research Center, Kermanshah University of Medical Sciences, Iran. The study included HCs with good sleep quality and Pittsburgh Sleep Quality Index (PSQI) scores under five. Moreover, patients with Par-I were identified based on the mismatch between PSQI scores and nocturnal polysomnography (PSG) measurements. The reported mismatch involves a variance of at least 1 h difference for total sleep time (TST) or 15% for sleep efficiency (SE) between objective (PSG) and subjective (PSQI) measures besides ID patients with objective TST and SE above 6 h, 30 min, and 85%, respectively (6). The exclusion criteria were pregnancy, a history of other neurological and mental illnesses, and MRI imaging contraindications such as having metal in their bodies or claustrophobia. The Kermanshah University of Medical Science Ethics Committee approved the study, and all participants signed informed consent before participating. After excluding five patients with mild obstructive sleep apnea, two patients with comorbid periodic leg movements, two patients with brain tumors, one patient with hydrocephaly, two Par-I patients, and four HC due to excessive head motions (translation > 1.5 mm and rotation > 1.5 degrees), the analysis included 15 Par-I patients (11 male, mean + SD age: 42.33 ± 12.23) and 48 HCs (24 male, age: 40.35 ± 12.67). The acquisition of images and the preprocessing of functional images were described in (14). Finally, ALFF maps were calculated for each Par-I and HC.
A chi-square test was conducted to assess potential gender differences between the two groups. The t-test was performed to calculate differences between age, PSQI, and head motion differences between the groups. Moreover, a t-test was used to check the possibility of ALFF differences between Par-I and HC. The effects of age and gender were controlled using a regressor, and multiple comparisons and the family-wise error (FWE) were used to prevent arbitrary results.
4. Results and Discussion
A total of 48 HC subjects (24 male, mean + SD (age): 40.35 ± 12.67) and 15 Par-I patients (11 male, age: 42.33 ± 12.23) were included in the study. Table 1 presents the demographic characteristics of all subjects. Additionally, a t-test was used to examine possible differences between Par-I and HC groups, and no significant differences were found (P-value = 1.000). Moreover, the result of the chi-square test indicated no significant differences in gender between the two groups (P-value = 0.112). Furthermore, t-tests revealed significant differences between the two groups in PSQI (P = 0.001), but not in head motion (P = 1.000) (Table 1).
| Variables | HC (48) | PDI (15) | P-Value |
|---|---|---|---|
| Gender (F, M) | 24, 24 | 4, 11 | 0.112 |
| Age | 40.35 ± 12.67 | 42.33 ± 12.23 | 1.000 |
| Head motion | 0.091 ± 0.052 | 0.088 ± 0.052 | 1.000 |
| PSQI | 3.33 ± 1.79 | 16.47 ± 2.03 | < 0.001 |
Abbreviation: PSQI, Pittsburgh sleep quality index.
a Values are expressed as mean ± SD unless otherwise indicated.
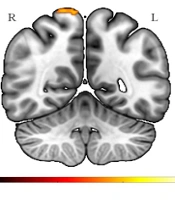
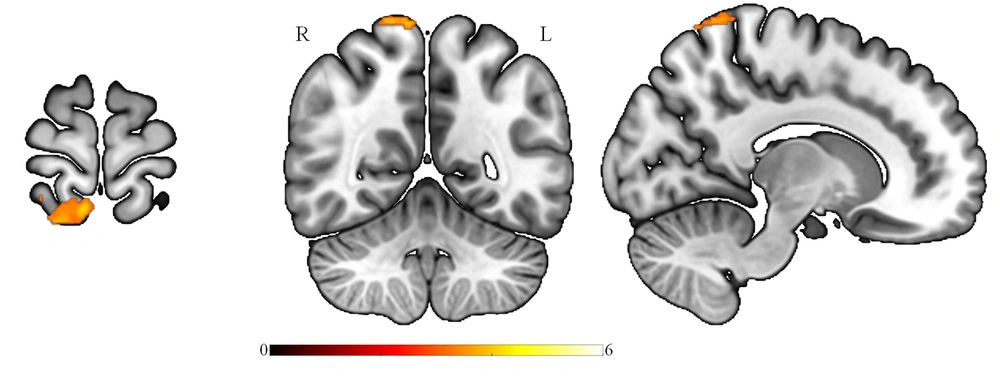
This study evaluated possible ALFF differences between patients with Par-I and HCs using the t-test. Multiple comparison corrections (FWE) revealed that ALFF decreased in superior parietal and precuneus regions in Par-I patients as compared to HC patients (Figure 1). The results were significant at the level of Pfwe < 0.001, and no significant differences were found in increased ALFF for Par-I compared to HC after multiple comparisons.
Survived significant brain regions after corrected for multiple comparisons, FWE correction, showed the amplitude of low-frequency fluctuation of paradoxical patients was less than healthy subjects at the superior parietal lobule and precuneus (MNIx, y, z: 8, -52, 74; cluster size: 156; PFWE < 0.001). FEW: Family wise error.
The purpose of this study was to assess whether ALFF differs between patients with Par-I and HC, based on resting-state fMRI images. The results indicated a significant ALFF decrease in the Par-I group compared to the healthy group. This decrease was evident in the superior parietal lobule and precuneus regions of the brain, which is in line with previous studies.
Koenigs et al. examined the role of parietal lobe regions in human working memory (15). Based on an extensive assessment of cognitive function and a sample of 19 patients with changes in the shape of the parietal lobe regions, they found that: (1) changes in the parietal lobe regions are related to deficits in information manipulation, and reorganization; (2) patients with parietal lobe changes have difficulty manipulating and reorganizing information in working memory for auditory-verbal and visual-spatial stimuli. Based on their findings, the parietal lobe regions are essential to manipulating and reorganizing information in working memory. In addition, previous studies have indicated the role and effects of sleep on memory areas (16). Furthermore, Li et al. investigated the functional relationship between brain regions among people with insomnia and HC and indicated changes in the functional connection between the parietal lobe and other brain parts compared to HC (17).
This network is assumed to be critical in regulating conscious processes, awareness, mental self-representation, and inferential information processing. Moreover, Levenson et al. found changes in these areas in insomnia disorder (18), as well as a relationship between precuneus structural and variation in brain function with the quality of sleep among depressive patients (19). Finally, further studies should investigate the role of this network in Par-I by patients with equal total sleep time due to the uncovered role of the parietal lobe in organizing memory and relation between impaired memory and Par-I (3, 20).
This study should be interpreted with caution due to the low number of included subjects. The leak of this information prevented us from analyzing other covariates, such as anxiety and depression factors, in order to unravel the pathophysiology of the parietal lobe. Furthermore, a 1.5 T scanner was used, while nowadays, scanners with 3T have been used to increase the signal-to-noise ratio and precision.
4.1. Conclusions
According to our findings, brain signals play a differential role in paradoxical insomnia's pathophysiology, and these findings may lead to further analysis of the underlying mechanism in the near future. Additionally, future studies should consider more complicated methods like machine learning with more subjects to study ID subtypes.