1. Background
Colon cancer is one of the most common and malignant cancers in men and women worldwide. Despite remarkable advances in diagnosing and treating colon cancer, the disease incidence is on the rise worldwide. Approximately 70 - 90% of colon cancers are related to lifestyle, physical activity, and dietary factors (1-4), and treatment strategies are available to fight cancer. On the other hand, there is no definitive cure for many types of cancer. Therefore, scientists decided to prioritize the move toward cancer prevention. The utilization of plants and their purified substances is prevalent, and many studied plants have been influential in treating cancer (2). This high potential of plants and their effective substances in treating and preventing cancer has led many scientists to study anti-cancer plants and seek to extract and identify their effective compounds worldwide, which are abundant in fruits, vegetables, grains, and cereals (5, 6). Many studies have shown the cytotoxic properties of oils, which increase treatment efficacy, reduce side effects, induce apoptosis, inhibit metastasis and angiogenesis in cancer cells, and decline drug resistance. Oils can be used alone or in combination with conventional cancer therapies (7, 8).
Pycnocycla belongs to the Apiaceae family, Apioideae subfamily, and Echinophora tribe. Pycnocycla includes 20 perennial, herbaceous, multicaulis, and spinous species in tropical and subtropical regions. About eight species of Pycnocycla, including Pycnocycla bashagardiana, are endemic to southern Iran and utilized in traditional medicine (9). The chemical composition analysis of the P. bashagardiana fruit essential oil (PBFEO) by Chehrazimeydan and Asgarpanah revealed the presence of myristicin (73.7%) as the major component (10). This finding characterized fruit oil as a rich natural source of myristicin as a phenylpropanoid involved in several cellular processes such as cytotoxic activities (11, 12). There are limited studies on the cytotoxic effects of the P. bashagardiana fruit essence in colon cancer cell lines, and the standard treatment of colon cancer is surgery. In this regard, the Pycnocycla fruit essence can probably inhibit cancer cells and be effective.
2. Objectives
This research investigated the cytotoxic effects of the P. bashagardiana fruit essence in the colon cancer cell line using cell culture and flow cytometry methods.
3. Methods
3.1. Preparation of Pycnocycla bashagardiana
The P. bashagardiana fruits were collected from Bashagard Village, Jask County, Hormozgan Province, Iran (25°38’38” N, 57°46’28” E, 900 m) in September 2017. The ground-dried aerial parts of the plant were subjected to hydrodistillation in a Clevenger-type apparatus for 4 h. At the end of distillation, the ES was collected, dried with anhydrous Na2SO4, measured, transferred to a clean glass vial, and kept at an appropriate temperature until performing biological and analytical tests (9, 10).
3.2. Pycnocycla bashagardiana Analysis
The studied oil was analyzed using the gas chromatography-mass spectrometry technique to identify the potentially responsible compounds for the observed pharmacologic activities. The oil sample was examined using an HP-6890 GC device with an FID and a DB-5 capillary column. The quantitative data were collected through the electronic integration of the FID peak areas. The oil components were determined by the retention time and indicators of C9-C28 n-alkanes, computer matching with the WILEY275.L library, and comparison of the mass spectra of the components with those of original samples with previous works. The composition percentages of the detected compounds were estimated by the GC peaks areas without correction factors and computed relative to each other (9, 10).
3.3. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assay
About 6000 cells with Roswell Park Memorial Institute (RPMI) and 10% phosphate-buffered saline FBS medium were cultured in each well of a 96-well plate to perform the experiments. The cells were exposed to 12.5, 25, 50, 100, 250, and 500 μg/mL within 24 and 48 h after one day of incubation at 37°C. Control wells were selected as untreated with 20 mL of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) solution (5 mg/mL) added at the end of treatment. The optical density of samples was read by an enzyme-linked immunosorbent assay reader at 570 nm after 15 min of incubation at room temperature and complete dissolution of the crystals (13-15).
3.4. Apoptosis Assay
The apoptosis and necrosis percentages were analyzed using the Annexin-V-FLUOS pI kit. The cells were incubated in a 25 cm2 plate in the RPMI medium. Then, concentrations lower than the half maximal inhibitory concentration (IC50) were selected from the results of the MTT method and added to the wells with three replications and incubated for 48 h. The cells were harvested with trypsin and collected after the treatment. Then, annexin-V-FLUOS labeled reagent solution, buffer, and propidium iodide (pI) solution (Sigma Aldrich, North America) were added to the target groups and incubated for 0.5h in the dark. Finally, 700 μL of the buffer was added to the samples and read by flow cytometry (FACSCalibur) and analyzed via FlowJO7.6.1 (2, 16).
3.5. Total RNA Extraction and Quantitative Real-time Polymerase Chain Reaction
The cells were cultured at 25000 to evaluate gene expression and treated at a defined concentration for 48 h after 24 h. Cellular RNA was extracted with the Yekta Tajhiz kit (Super RNA Extraction kit for Tissue & Cells, Cat: YT9080) based on the kit protocol. All real-time polymerase chain reactions (RT-PCRs) were performed in an ABI step-one system. The gene expression was evaluated using the SYBER Green quantitative polymerase chain reaction (qPCR) Master MIX (Amplicon) kit and performed at 95, 60, and 72°C for 15, 30, and 15 s, respectively. The gene expression analysis was evaluated with 2-∆∆Ct (Livak) (Schmittgen Th, 2008). The gene primer sequences are shown in Table 1.
| Gene and Primer Sequence | Product Size (bp) |
|---|---|
| BAX | 169 |
| 5’TTGCTTCAGGGTTTCATCCAG3’ | |
| 5’AGCTTCTTGGTGGACGCATCC3’ | |
| BCL2 | 170 |
| 5’TGTGGATGACTGAGTACCTGAACC3’ | |
| 5’CAGCCAGGAGAAATCAAACAGAG3’ | |
| GAPDH | 139 |
| 5’CCACTCCTCCACCTTTGACGCT3’ | |
| 5’TTACTCCTTGGAGGCCATGTGG3’ |
Primer Sequences for Real-time Reverse-transcription Polymerase Chain Reaction Analysis
3.6. Molecular Docking Studies
A primary target in molecular docking was the susceptibility to evaluate the scoring function and estimate protein-ligand interactions to predict the activity and binding affinity of the ligand molecule. The BCL2 protein sequence was taken from the UniProt site, modeled by the Swiss model, and minimized by Swiss-pdbViewer software. The myristicin structure was downloaded in an SDF format from the PubChem site and converted to a PDB format by OPENABLE. The efficacy and binding of the myristicin ligand to the BCL2 protein were performed using AutoDock Vina software. ABT-199 was considered as a control and a clinical inhibitor of the BCL2 protein to compare the results of this study.
3.7. Statistical Analysis
GraphPad Prism software was used to analyze the results, draw charts, and compare the groups with one-way ANOVA and Tukey’s post hoc test at a significance level of P < 0.05.
4. Results
4.1. Pycnocycla bashagardiana Analysis
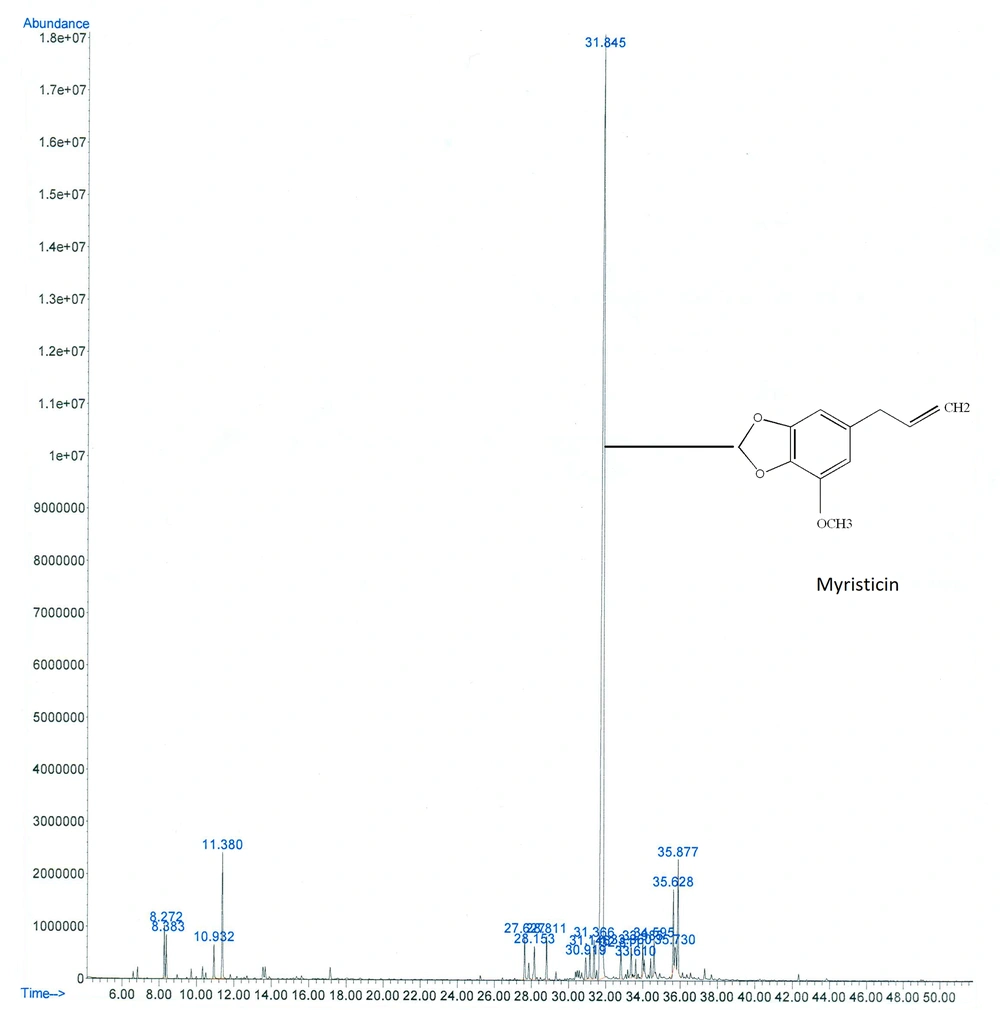
The hydrodistillation of P. bashagardiana fruits generated a yellowish oil with a strong odor and 0.8% (v/w) yield based on the fresh weight. Figure 1 shows the gas chromatogram of PBFEO analysis. Based on Table 2, 12 compounds were specified in the oil, accounting for almost 98.7% of the total chromatographical material, and myristicin (75.1%) was the main oil constituent.
| Compound a | KI b | KI c | Percentage |
|---|---|---|---|
| 1. Sabinene | 972 | 975 | 1.3 |
| 2. β-Pinene | 976 | 979 | 7.9 |
| 3. Z-β-Ocimene | 1035 | 1037 | 1.4 |
| 4. E-β-Ocimene | 1052 | 1050 | 3.8 |
| 5. β-Cubebene | 1394 | 1391 | 1.6 |
| 6. Methyl eugenol | 1396 | 1401 | 0.9 |
| 7. α-Guaiene | 1445 | 1440 | 1.2 |
| 8. δ-Guaiene | 1505 | 1508 | 1.1 |
| 9. Myristicin | 1523 | 1520 | 75.1 |
| 10. Caryophyllene oxide | 1588 | 1583 | 0.4 |
| 11. Isomyristicin | 1627 | 1624 | 1.5 |
| 12. β-Eudesmol | 1655 | 1651 | 2.5 |
| Total | 98.7 |
Gas Chromatography-mass Spectrometry Analysis of Pycnocycla bashagardiana Fruit Essential Oil
4.2. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Measurement
Figure 2 shows the viability rates of PBFEO-treated normal L929 and HT29 cancer cells decreased at 0 - 500 μg/mL in 24 and 48 h. The viability decrease in normal L929 cells was significant only at 500 μg/mL compared with the control group (P ≤ 0.0001), and the highest (99.733 and 85.729%) and lowest (41.422 and 44.713%) viability rates were shown at 12.5 and 500 μg/mL in 24 and 48 h, respectively. The IC50 concentrations of 431.8 and 450 μg/mL were recorded in 24 and 48 h, respectively. The highest (99.9 and 90.65%) and lowest (38.371 and 21.068%) viability rates in HT29 cancer cells were found at 12.5 and 500 μg/mL in 24 and 48 h, respectively. The IC50 concentrations of 278.5 and 144.6 μg/mL were detected in 24 and 48 h, respectively (15, 17).
Comparison of the effects of Pycnocycla bashagardiana fruit essential oil (PBFEO) concentrations on the viability of L929 and HT29 cell lines at 24 and 48 h. The results are presented as the means ± standard deviation (SD). * P < 0.05, * P < 0.01, *** P < 0.001, **** P < 0.001 compared with the control group
4.3. Cell Apoptosis
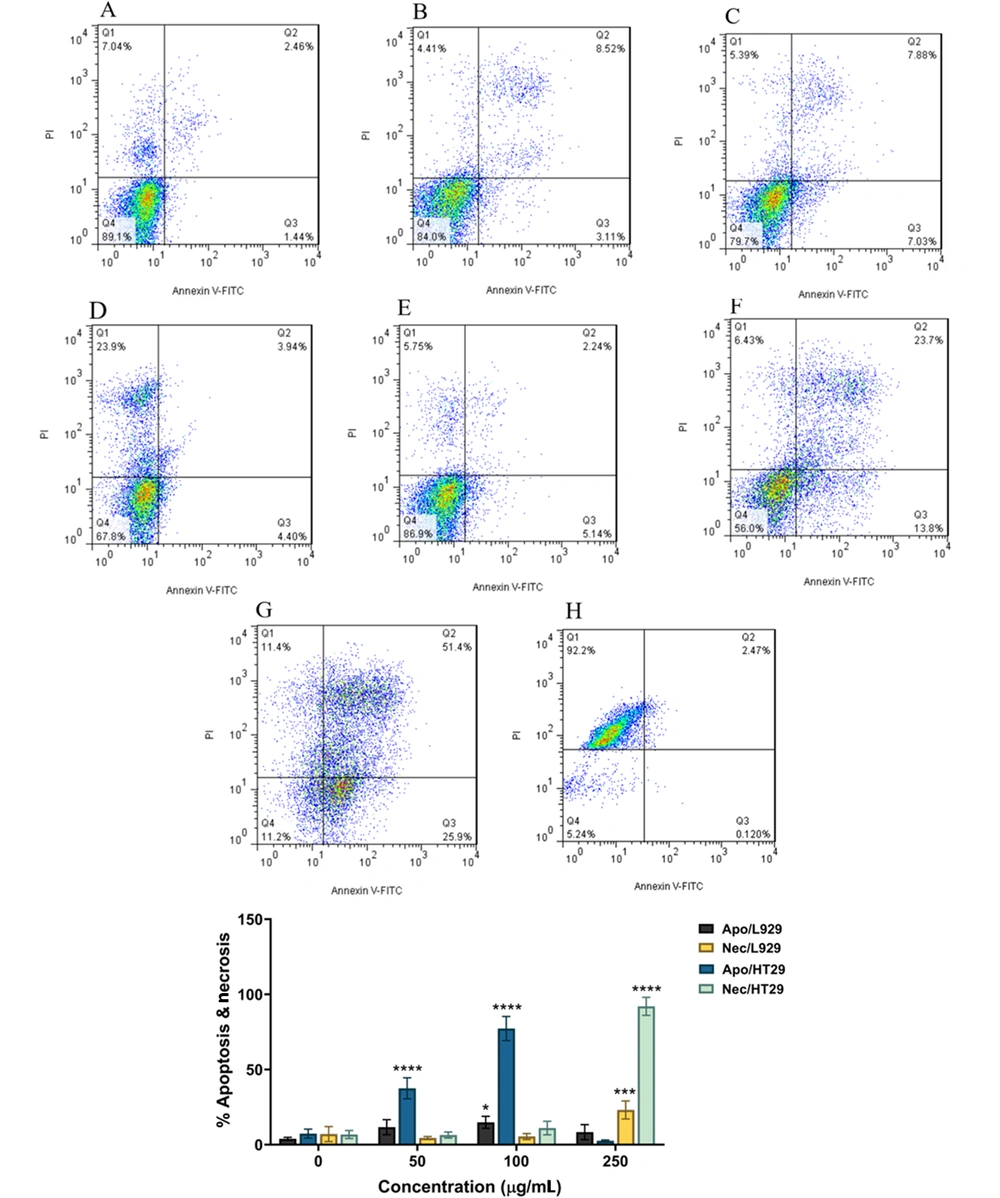
Based on the MTT test results, the apoptosis percentages of L929 and HT29 cancer cell lines treated with PBFEO were tested at three concentrations of 50, 100, and 250 μg/mL in 48 h. An increase in apoptosis was observed in normal L929 cells treated with PBFEO at 50 (2.9 fold), 100 (3.8 fold), and 250 μg/mL (2.1 fold) compared with untreated cells. Further, a significant difference was detected only at 100 μg/mL compared with untreated cells (P < 0.05). A significant increase was shown in necrosis at 250 μg/mL (P < 0.001). Apoptosis in HT29 cancer cells treated with PBFEO at 50 and 100 g/mL increased 5.08 and 10.4 times with a significant difference from untreated cells. Furthermore, significant necrosis was detected compared with the untreated cells at 250 μg/mL (P < 0.0001). The apoptosis rates resulting from treating HT29 cancer cells and normal L929 cells at 50 and 100 μg/mL of PBFEO were compared by statistical analyses. Significant (3.2% (P < 0.001) and 5.1 (P < 0.0001)) increases were observed in the apoptosis rate in HT29 cancer cells compared with L929 cells at 50 and 100 μg/mL of PBFEO, respectively.
4.4. The Expression of BCL2 and BAX Genes
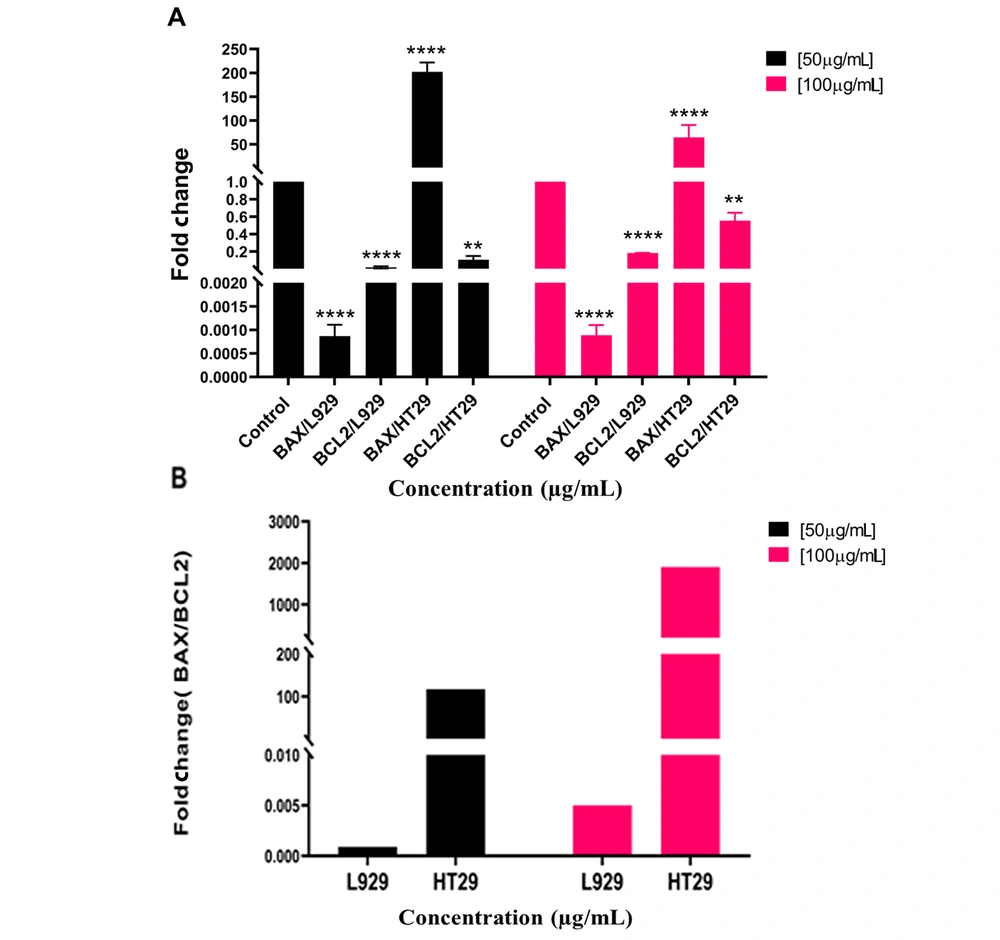
As shown in Figure 3, there are a significant decrease in normal L929 cells treated with PBFEO in fold changes and relative expression of the BCL2 gene at 50 (P < 0.0001) and 100 μg/mL (P < 0.01), respectively. The fold changes in the relative expression of the BAX gene significantly decreased at 50 and 100 μg/mL compared to the control (P < 0.0001). A significant decrease occurred in the fold change at 50 μg/mL (P < 0.01), which non-significantly reduced the relative expression of the BCL2 gene in PBFEO-treated HT29 cancer cells at 100 μg/mL. On the other hand, significant increases were found in the relative expression of the BAX gene at 50 and 100 μg/mL (P < 0.0001) compared with untreated cells. Based on Figure 3, the BAX/BCL2 ratios decrease in the normal L929 cell treated with PBFEO at 50 and 100 μg/mL compared to the HT29 colon cancer cell line.
Changes in the expression of BAX and BCL2 genes in the L929 and HT29 cell lines treated with Pycnocycla bashagardiana fruit essential oil (PBFEO) at 50 and 100 μg/mL for 48 h; A, * Denotes significance relative to the control group, ** P < 0.01 and **** P < 0.0001 compared with the control group; B, BAX/BCL2 gene expression ratio in L929 and HT29 cell lines treated with PBFEO at 50 and 100 μg/mL for 48 h.
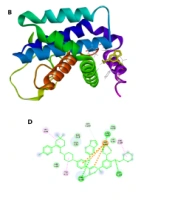
4.5. Protein-Ligand Docking
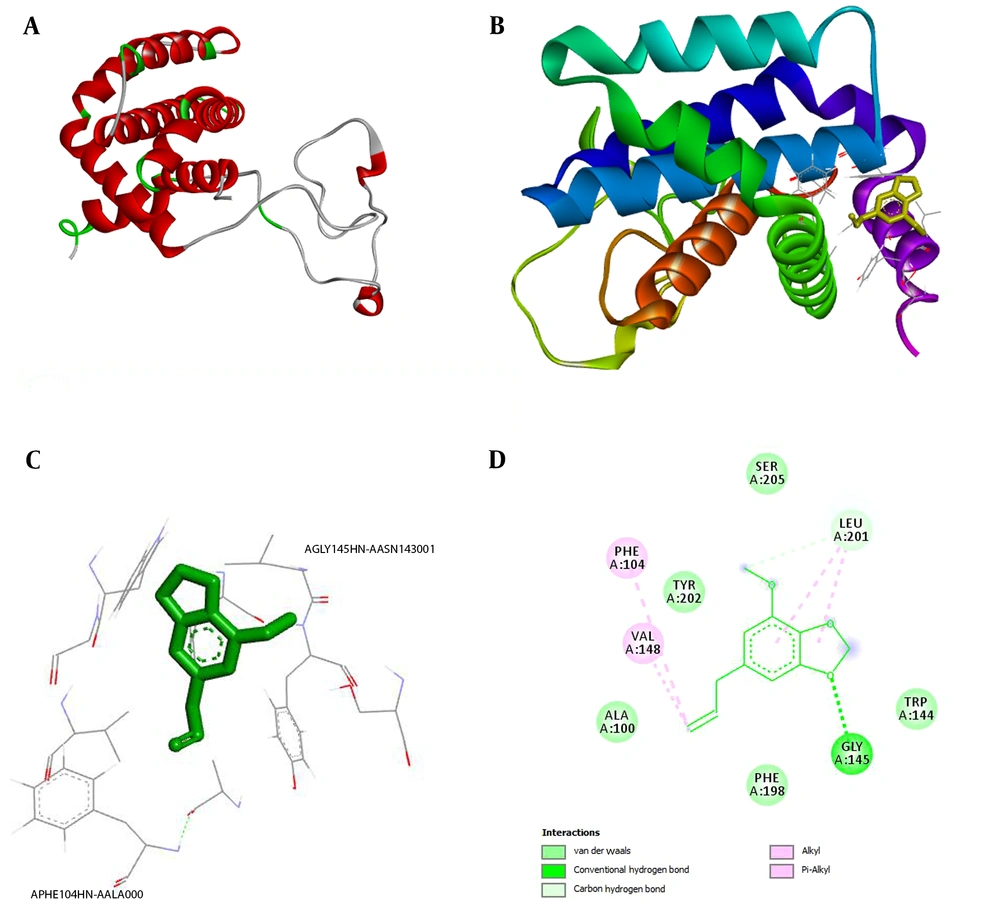
Figure 4A depicts the BCL2 protein, and Figure 4B indicates the efficacy and binding of the myristicin ligand to the BCL2 protein. H-bonding interactions between the myristicin ligand and BCL2 protein target are illustrated in Figure 4C. Val93, Leu97, Asp103, Phe104, Arg106, and Arg107 amino acids were considered as the binding sites for the BCL2 protein. The binding energy of the myristicin ligand to the BCL2 protein is numerically equal to -5.3 (energy binding for ABT-199 equals -7.5). Van der Waals forces were observed in the A: GLY145: H and A: ASN143: OD1 region of the BCL2 protein. As a result, the ligand binding to the protein can occur in this region. Although myristicin has a weaker binding affinity than the control, it is still acceptable.
5. Discussion
The effect of PBFEO fruit essence on reducing the proliferation of colon cancer cells remains a controversial topic despite the ability of the PBFEO plant to inhibit cancer cells (11, 12). Accordingly, the current study used MTT assay, flow cytometry, and RT-PCR methods to investigate the cytotoxic effects of the PBFEO fruit essence. The study demonstrated that PBFEO fruit essence could destroy colon cancer cells through cellular and molecular mechanisms. In this regard, plants rich in metabolites such as alkaloids, flavonoids, and terpenoids were focused on their historical records in cancer treatment (5, 18-20). The results demonstrated that myristicin is the main component of the oil (75.1%). Several studies have explored myristicin and myristicin-containing herbal extracts’ anti-inflammatory, anti-cancer, and apoptotic effects in animal models and cancer cell lines. The mitochondria pathway plays a role in causing apoptosis by activating caspase enzymes. The BCL2 protein family is a regulator of mitochondria, which is one of the essential anti-apoptotic proteins in living cells. BAX is considered as an inactive protein in the cytosol or membrane as a monomer. The increased BAX expression compared to BCL2 activated the caspase cascade and inducing apoptosis in apoptotic cells (19, 20). Pycnocycla bashagardiana fruit essential oil was evaluated on the HT29 cancer cell line and the L929 cancer cell line for its antiproliferative effect and apoptosis induction. In agreement with these findings, a study reported that the EO prepared from Myristica fragrans contained 32.8% myristicin, which inhibited the growth of CaCO2 cancer cells (18). Decreased cell viability was observed in cancerous and normal cells after treatment with different concentrations of PBFEO.
Moreover, the inhibition of cell proliferation is concentration-dependent. A significant increase in apoptosis was found in HT29 cancer cells compared to the control (untreated). The effects of the mace extract from M. fragrans against Helicobacter pylori as an anti-inflammatory property were reported on the RAW264.7 cell line. Additionally, antioxidant and anti-cancer effects were found on the KATO III gastric cancer cell line (20). The researchers have observed decreased cell viability, accumulated cytochrome c, activated CASPASE 3, and induced apoptosis (21). The carcinoma KB cell line treated with the M. fragrans mace extract could inhibit cancer cells and reduce the expression of the BCL2 gene (22). Another study confirmed the anti-violence effect of P. bagardiana EO on male Wistar rats (23). In this study, the gene expression results revealed a significant increase in the relative expression ratio of BAX/BCL2 genes in cancer cells compared to normal cells. The rise in BAX/BCL2 gene ratio had a crucial role in the mitochondria in PBFEO-induced apoptosis. Zhang et al. as cited in Zengin et al. demonstrated the antimicrobial effects of P. peucedanifolia and P. ferulacea plants on Staphylococcus aureus and Bacillus cereus as Gram-positive bacterial strains. The antioxidant and cytotoxic effects were reported on the HepG2 hepatocarcinoma cell line and murine bone marrow stromal cell line S17 (24). According to previous evidence, EOs did not have a specific cellular target, crossing the cell membrane and causing damage to the membrane due to their lipophilic properties. Essential oils (EOs) can be used as an antitumor in treating cancers due to their peroxidation property, mitochondrial function intervention, free radical production, and lack of side effects on healthy tissues (25).
A study confirmed the cytotoxic and genotoxic effects of myristicin and 1’-hydroxymyristicin after exposure to HepG2 cells for 24 h (26). Another study investigated the cytotoxic and apoptotic effects of myristicin on SK-N-SH human neuroblastoma cells and reported that this compound could cause apoptosis in this cell line by cytotoxicity (21). In the present study, myristicin ligand binding to the BCL2 protein was analyzed using molecular docking analysis. The weak binding affinity of myristicin to BCL2 was reported compared to the control, which can be considered a factor in inducing fewer side effects in healthy cells. Therefore, diets with EOs rich in myristicin were considered as a cancer treatment.
Further extensive in vitro and in vivo studies are needed to better investigate the anti-cancer effects of PBFEO. However, more extensive research is necessary to accurately analyze the effects of the PBFEO fruit EO on colon cancer cells. This study evaluated the cytotoxic effects of the PBFEO fruit EO on colon cancer cells and fibroblast cells in the cell culture. This research could clarify the relationship between the PBFEO fruit EO and colon cancer to manage and prevent liver cancer.
5.1. Conclusions
The results showed the decreased viability rates of normal L929 and HT29 cancer cells treated with different PBFEO concentrations. On the other hand, HT29 cancer cells showed an increase in apoptosis and BAX/BCL2 gene expression compared with normal L929 cells. The cytotoxic and apoptotic effects were observed by treating HT29 cancer cells with PBFEO and the weak binding efficiency of myristicin to the BCL2 protein, which can be suggested as a complementary treatment in colon cancer through more effective treatment and a decline in the side effects of normal cells.