1. Background
Breast cancer is the most common cancer in women worldwide (1). The incidence rate has increased in recent decades in both developed and undeveloped countries (2). In addition, the survival rate of patients has increased in recent years (3). Early diagnosis due to screening with digital mammography and progress in treatment modalities, including more effective systemic treatments, can improve the patients’ survival (4). According to the World Health Organization (WHO) statistics in Asia, the age-standardized breast cancer incidence and mortality rates were estimated at 29.1 and 10.2 per 100 000 persons, respectively (5). Patients’ survival rates and epidemiological characteristics vary in different regions of Asia (6). In addition, it seems that breast cancer incidence and survival rates in other provinces of Iran vary widely depending on their economic status (7, 8). Breast cancer patients’ characteristics and treatment results in the southeast of Iran have not been comprehensively investigated.
This study evaluated patients' characteristics and survival rates and compared them with those of other studies. The prevalence of intrinsic luminal subtypes and their prognostic significance in Iranian patients have not yet been reported. The axillary staging modality was not considered in previous studies conducted in Iran. This evaluation is essential due to the novelty of sentinel biopsy in Iran. Further, prognothe sis is rarely influenced by the percentage of involved nodes.
2. Methods
This research was approved by the Kerman University of Medical Science Ethics Committee (IR.KMU.AH.REC.1397.090). This cohort was retrospectively performed on invasive breast cancer patients treated in the Radiation Oncology Department, Kerman University of Medical Sciences, from March 2004 to March 2020. The recorded clinical and pathological information was used for analysis. Whenever necessary, the patient was contacted to complete the form, and those patients with no regular follow-up or incomplete treatment were excluded. The data related to the patients’ characteristics [age, tumor (T) stage, nodal (N) stage, metastasis (M), group staging, immunohistochemistry (IHC) study for estrogen (ER) and progesterone (PR) receptor, Ki67 and Her2/neu, and the percentage of the involved dissected lymph nodes (PIDNs)] were extracted. Moreover, the treatment-related information was extracted, including surgery type, axillary staging type (standard dissection vs. sentinel biopsy), receiving or not receiving radiotherapy, chemotherapy, and hormonal treatment. The American Joint Committee on Cancer (AJCC) staging version 8 was used for staging. Those patients with ER- and PR-positive, Her2-negative, and low Ki67 expression levels were considered luminal A. For luminal B, ER-positive, Her2-negative, and PR-negative or high Ki67 were considered. Her2-enriched cases were those with ER- and PR-negative, and Her2-positive staining.
The triple-negative group was referred to as those with ER-/PR- and Her2-negative IHC.
Three-, 5-, and 10-year overall survival (OS) rates were estimated by the Kaplan-Meier method. For this purpose, the dates of the first and last visits were used. Disease-free survival (DFS) was calculated for those without metastasis at presentation. These patients were censored for either locoregional or distant recurrence. Progression-free survival was estimated for metastatic patients. The recorded date of clinical or radiological progression was used for this purpose. The life table was considered for OS and DFS estimation. A log-rank test was used to determine the difference in survival rates based on various factors. Qualitative variables were described using numbers and percentages, and the age distribution was reported using means and SDs. The first step was to use a simple Cox regression model. The variables with P values lower than 0.2 (except age, which was included in all models) were considered in multiple Cox regression using three modeling strategies.
- Model 1: Age, locoregional recurrence, axillary staging type, group stage, and luminal subtype were included.
- Model 2: The group stage was replaced with its component (i.e., T-stage, N-stage, and M-stage).
- Model 3: PIDN was added to Model 2.
A proportional hazard test and log-minus-log plots were used to check the proportional hazard assumption for each model. P values less than 0.05 were considered statistically significant. The data analysis was performed using SPSS version 19 (SPSS Inc., Chicago, Ill, USA).
3. Results
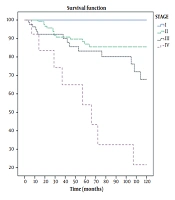
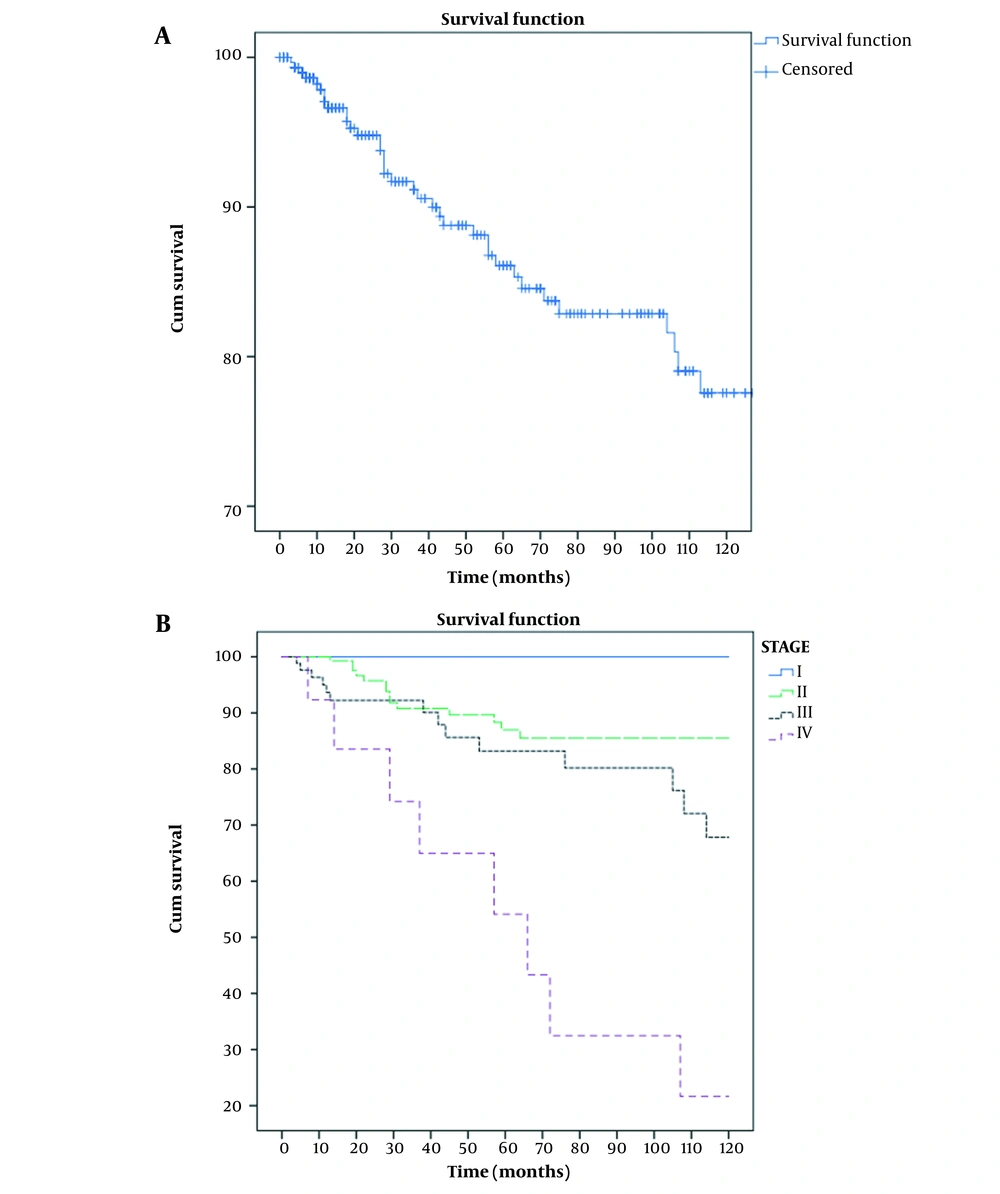
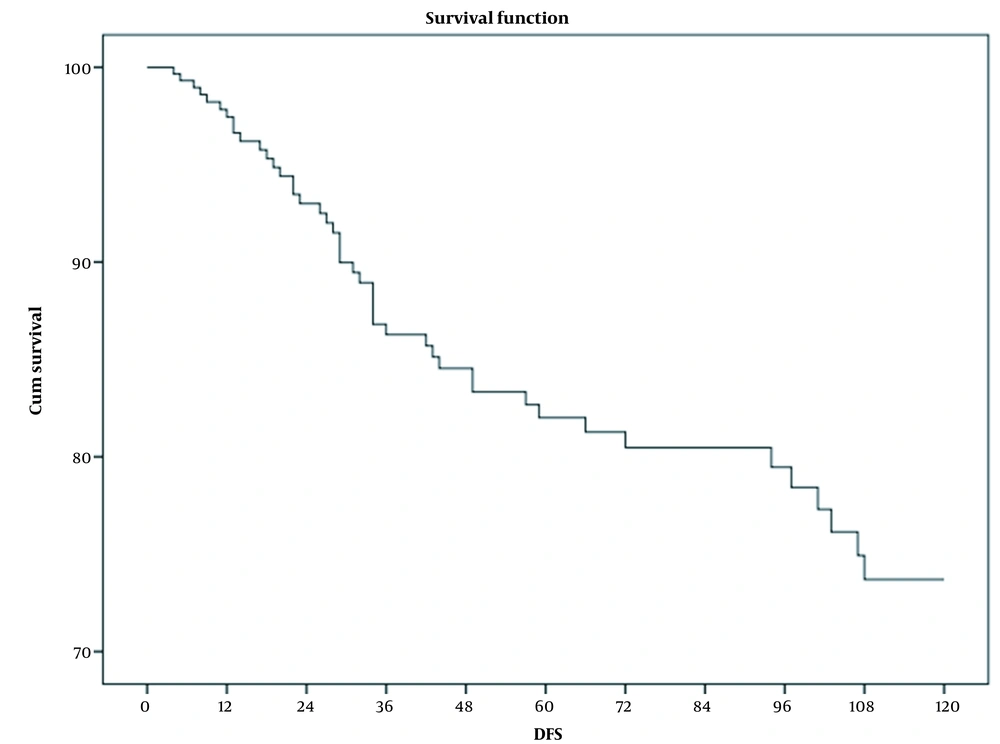
This study included 309 patients out of 316 who met the inclusion criteria. The mean follow-up time was 56.4 months (from 4 to 224 months; SD, 49.5). Table 1 shows the demographic characteristics and treatment details of the patients. Among 309 patients in the cohort, 40 (12.9%) deaths were identified: 15 in stage II, 16 in stage III, and 9 in stage IV. No death was observed in stage I. About 62% of dead patients had more than 50% involved nodes. The total mortality rate was 28 per 1000 person-years (95% CI, 20 - 37) for all patients, 20 per 1000 person-years (95% CI, 12 - 33) in stage II, 41 per 1000 person-years (95% CI, 25 - 67) in stage III, and 151 per 1000 person-years (95% CI, 79 - 292) in stage IV. The mean survival time was 14.5 (95% CI, 13.4 - 15.8) years for all patients, as well as 15.9 (95% CI, 14.6 - 17.3), 10.8 (95% CI, 8.8 - 12.9), and 5.9 (95% CI, 3.4 - 8.4) years for stages II, III, and IV, respectively. Three-year OS rates were 91%, 100%, 91%, 92%, and 65% for all patients and stages I, II, III, and IV, respectively. Five-year OS rates for all patients and stages I, II, III, and IV were 86%, 100%, 87%, 83%, and 54%, respectively. Ten-year OS rates for all patients and stages I, II, III, and IV were 63%, 100%, 77%, 43%, and 22%, respectively (Figure 1). Three-, 5-, and 10-year DFS rates for nonmetastatic patients were 86%, 82%, and 60%, respectively (Figure 2). During the follow-up period, locoregional recurrence and new metastasis occurred in 9 (2.9%) and 52 (16.8%) patients. The metastatic sites included the lung (18 patients), bone (14 patients), brain (8 patients), liver (6 patients), and multiple sites (6 patients). Two-, 3-, and 5-year progression-free survival rates for metastatic patients were 56%, 44%, and 13%, respectively. The mean progression time was 34.3 months (95% CI, 22.3 - 46; SD, 6.1). Table 2 shows the average survival time based on various factors. A log-rank test revealed a significant correlation between the stage, luminal subtype, hormone therapy, and percentage of positive dissected nodes. Unadjusted Cox regression also showed a significant association between the stage (group stage, T-stage, N- stage, and M-stage), luminal subtype, and PIDN with survival. In contrast, no correlation was found among age, surgery type, locoregional recurrence, axillary staging type, and receiving or not radiotherapy (Table 3). According to the Cox regression model, patients with more than 50% involved dissected lymph nodes had a lower survival rate than those with less than 50% involved nodes. In addition, a lower survival rate was observed in luminal B, Her2 enrich, and triple-negative patients compared with luminal A patients. Among the five factors included in multivariable model 1, only the group stage and luminal subtype were significantly correlated with survival and age. locoregional recurrence and axillary staging modality showed no significant relationship. Patients in stages III and IV had lower survival rates than those in stage II. The replacing group, stage by its component in model 2, showed that T-stage (T3-T4 compared to T1-T2) and M-stage had a significant relationship with survival, but N-stage had no significant correlation. PIDN adjustment reduced the association between metastasis and survival in model 3 (Table 4). Covariate analysis revealed that group stage, T-stage, M-stage, luminal subtype, PIDN, and locoregional recurrence were independent prognostic factors. The proportional hazard assumptions of the model were not found to be violated.
| Characters | Findings (309 Patients) b |
|---|---|
| Age (y); mean ± SD (range) | 51.1 ± 13.6 (23 - 87) |
| ≤ 50 | 153 (49.5) |
| > 50 | 156 (50.5) |
| Stage (pathologic) | |
| T stage | T1:33 (10.7); T2:168 (54.4); T3:80 (25.9); T4:28 (9.1) |
| N stage | N0: 162 (52.4); N1: 82 (26.5); N2: 37 (12); N3: 28 (9.1) |
| M-stage | M1: 296 (95.8); M0: 13 (4.2) |
| Group stage | I: 37 (12); II: 169 (54.7); III: 90 (29.1); IV: 13 (4.2) |
| IHC | |
| ER | +ve: 144 (46.6); -ve:89 (28.8); Unknown:76 (24.6) |
| PR | +ve:147 (47.6); -ve:88 (28.5); Unknown:74 (23.9) |
| Her2neu | +ve:118 (38.2); -ve:108 (35); Unknown:83 (26.9) |
| ki- 67 | Low:26 (8.4); High:193 (62.4); Unknown:90 (29.2) |
| Luminal | |
| A | 58 (18.8) |
| B | 107 (34.6) |
| Her2 enrich | 32 (10.4) |
| Triple -ve | 46 (14.9) |
| Unknown | 66 (21.4) |
| Surgery type | |
| Mastectomy | 135 ( 43.7) |
| Conservative | 174 (56.3) |
| Radiotherapy | |
| Yes | 227 (73.5) |
| No | 82 (26.5) |
| Chemotherapy | |
| Yes | 286 (92.6) |
| No | 23 (7.4) |
| Hormone therapy | |
| Yes | 158 (51.1) |
| No | 71 (23) |
| Unknown | 80 (25.9) |
| Axillary staging | |
| Dissection | 222 (71.8) |
| Sentinel biopsy | 87 (28.2) |
| Survival status | |
| Alive | 269 (87.1) |
| Dead | 40 (12.9) |
a Values are expressed as No. (%) unless otherwise indicated.
b Seven patients (2.2%) with no follow-up were excluded.
| Factors | The Mean Survival a ± SD (Range) | P-Value |
|---|---|---|
| Age (y) | 0.06 | |
| ≤ 50 | 197.9 ± 10.1 (178.1 - 217.8) | |
| > 50 | 140.7 ± 7.4 (126 - 155.4) | |
| Stage | ||
| T | 0.04 | |
| T1 | 201.2 ± 10 (181.4 - 221) | |
| T2 | 187.9 ± 8.8 (170.5 - 205) | |
| T3 | 134.5 ± 10.4 (114.1 - 154.9) | |
| T4 | 109.3 ± 12.2 (85.3 - 133.3) | |
| N | < 0.005 | |
| N0 | 220.1 ± 7.3 (187.7 - 216.4) | |
| N1 | 156.5 ± 10.9 (130.2 - 177.9) | |
| N2 | 110.9 ± 9.1 (93 - 128.8) | |
| N3 | 107.4 ± 13.3 (81.3 - 133.6) | |
| M | < 0.005 | |
| M0 | 184.2 ± 7 (170.4 - 198) | |
| M1 | 71 ± 15.2 (41.1 - 100.9) | |
| Group stage | < 0.005 | |
| Luminal | 0.002 | |
| A | 198.6 ± 8.3 (182.3 - 214.9) | |
| B | 179.2 ± 11.7 ( 156.2,202.2) | |
| Her2rich | 119.1 ± 14.8 (909 - 148.3) | |
| Triple-ve | 96.4 ± 10.8 (75.2 - 117.6) | |
| Surgery type | 0.23 | |
| Mastectomy | 159.5 ± 9.3 (141.2 - 177.7) | |
| Conservative | 178.3 ± 9.6 (159.4 - 197.1) | |
| Radiotherapy | 0.53 | |
| Yes | 178.5 ± 8.7 (161.3 - 195.7) | |
| No | 139.7 ± 8.2 (123.6 - 155.9) | |
| Chemotherapy | 0.94 | |
| Yes | 175.8 ± 7.5 (161 - 190.5) | |
| No | 156.7 ± 28.1 (101.4 - 211.9) | |
| Hormone therapy | 0.003 | |
| Yes | 184.5 ± 7.6 (169.5 - 199.4) | |
| No | 120.3 ± 12 (96.6 - 144) | |
| Locoregional recurrence | 0.12 | |
| Yes | 135.6 ± 28.6 (79.6 - 19.7) | |
| No | 178.4 ± 7.3 (163.9 - 192.9) | |
| Axillary staging | 0.08 | |
| Dissection | 158.2 ± 7.4 (143.5 - 172.8) | |
| Sentinel | 189.8 ± 11.1 (167.9 - 211.6) | |
| PIDN (%) | 0.003 | |
| ≤ 50 | 152.6 ± 6.1 (140.5 - 164.8) | |
| > 50 | 121.5 ± 13.6 (94.8 - 148.1) |
a Months.
| Variables and Categories | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 0.59 | |
| Continuous | 0.99 (0.97 - 1.02) | |
| Locoregional recurrence | 0.14 | |
| No | Reference | |
| Yes | 2.21 (0.77 - 6.31) | |
| PIDN | 0.002 | |
| ≤ 50 | Reference | |
| > 50 | 3.03 (1.51 - 6.08) | |
| Axillary staging | 0.09 | |
| Dissection | 1.97 (0.90 - 4.29) | |
| Sentinel | Reference | |
| T-stage | 0.008 | |
| T0-T1 | Reference | |
| T2-T3 | 2.32 (1.24 - 4.32) | |
| N-stage | 0.002 | |
| N0-N1 | Reference | |
| N2-N3 | 2.78 (1.47 - 5.26) | |
| M-stage | < 0.001 | |
| M0 | Reference | |
| M1 | 16.27 (7.74 - 34.19) | |
| Group stage | ||
| II | Reference | |
| III | 2.77 (1.37 - 5.63) | 0.005 |
| IV | 9.35 (4.09 - 21.39) | < 0.001 |
| Luminal subtype | ||
| Luminal A | Reference | |
| Luminal B | 2.82 (0.91 - 8.75) | 0.07 |
| Her2 enrich | 5.71 (1.74 - 18.72) | 0.004 |
| Triple-negative | 6.67 (2.07 - 21.48) | 0.001 |
| Surgery type | 0.25 | |
| Mastectomy | 1.44 (0.77 - 2.69) | |
| Conservative | Reference | |
| Radiotherapy | 0.55 | |
| No | 1.22 (0.64 - 2.34) | |
| Yes | Reference |
Abbreviation: PIDN, percentage of the involved dissected lymph nodes.
| Variables and Categories | Model 1 a | Model 2 a | Model 3 a | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 0.55 | |||||
| Continuous | 0.99 (0.97 - 1.02) | 0.65 | 1.00 (0.97 - 1.03) | 0.67 | 0.99 (0.96 - 1.02) | |
| Locoregional recurrence | 0.01 | |||||
| No | Reference | 0.61 | Reference | 0.63 | Reference | |
| Yes | 1.37 (0.40 - 4.70) | 1.33 (0.41 - 4.29) | 5.72 (1.39 - 23.61) | |||
| PIDN | 0.04 | |||||
| ≤ 50 | Not included | - | Not included | - | Reference | |
| > 50 | 2.43 (0.97 - 6.08) | |||||
| Axillary staging | 0.13 | |||||
| Dissection | 1.92 (0.74 - 4.95) | 0.18 | 2.84 (0.91 - 8.93) | 0.07 | 4.23 (0.76 - 23.53) | |
| Sentinel | Reference | Reference | Reference | |||
| T-stage | ||||||
| T0-T1 | Not included | - | Reference | 0.04 | Not included | - |
| T2-T3 | 2.36 (1.05 - 5.29) | |||||
| N-stage | ||||||
| N0-N1 | Not included | - | 0.70 (0.29 - 1.70) | 0.43 | Not included | - |
| N2-N3 | Reference | |||||
| M- stage | ||||||
| No | Reference | Reference | < 0.005 | |||
| Yes | Not included | - | 11.80 (5.24 - 26.58) | < 0.001 | 8.21 (3.47 - 19.41) | |
| Stage Group | ||||||
| II | Reference | Not included | - | Not included | - | |
| III | 2.90 (1.31 - 6.38) | 0.008 | ||||
| IV | 6.99 (2.55 - 19.16) | < 0.001 | ||||
| Luminal subtype | ||||||
| Luminal A | Reference | Reference | Reference | |||
| Luminal B | 3.33 (1.05 - 10.50) | 0.04 | 2.92 (0.93 - 9.18) | 0.07 | 2.46 (0.75 - 7.99) | 0.13 |
| Her two enrich | 4.41 (1.21 - 16.00) | 0.02 | 4.12 (1.10 - 15.39) | 0.04 | 2.38 (0.53 - 10.51) | 0.25 |
| Triple-negative | 5.21 (1.61 - 16.87) | 0.006 | 4.61 (1.41 - 15.01) | 0.01 | 3.62 (1.08 - 12.09) | 0.03 |
a Adjusted variables: Model 1, locoregional recurrence, axillary staging type, group stage, and luminal subtype; Model 2, the group stage was replaced by T-stage, N-stage, and M-stage; Model 3, the PIDN was added to model 2.
4. Discussion
The mean age of the patients was 51.1 years, which is consistent with previous studies (1, 9). However, almost 50% of the patients were under 51 years old. About 80% of Western patients are over 50 years old (9). In Arab nations, more than 60% of patients are under 50 years of age (10-13). The high percentage of young patients in non-Western countries is important in developing screening programs (13). Immunohistochemical and molecular subtypes are significant in the treatment and prognosis of breast cancer. Generally, luminal A, luminal B, Her2 enrich, and triple-negative subtypes account for 30% - 40%, 20% - 30%, 12% - 20%, and 15% - 20% of breast cancer cases, respectively (14). However, the prevalence of luminal A has been reported to be up to 77% (15). About 35% of the patients were classified as luminal B. Luminal A accounted for onlytients. The difference in the prevalence of different subtypes in studies can be due to selecting the variable cut-off points. For example, variable cut-off points were proposed to determine low Ki67 expression (from 10% to 20%) (16, 17). These differences should be considered in evaluating the role of molecular subtypes in survival. A cut-off was chosen of < 15%, as suggested by the St. Gallen International Expert Consensus, as a low expression (18). Five- and 10-year OS rates for our cohort were 86% and 63%, respectively. Five- and 10-year DFS rates were estimated at 82% and 60%, respectively. These survival results are consistent with other studies (7, 8). Several prognostic factors have been raised in breast cancer. In addition to the stage, various factors have been proposed (including age, lymphovascular invasion, molecular characteristics, tumor grade, and nodal status) (9). According to a log-rank test, age (≤ 50 years compared to > 50 years), stage (T-stage, N-stage, and group stage), luminal subtypes, hormone treatment, and PIDN had a significant relationship with survival. However, the relationship between N-stage and age with survival was insignificant based on multivariate analysis, and locoregional recurrence was correlated with decreased survival. Some studies have reported lower survival for younger patients, but the worse prognosis for younger patients is related to other prognostic factors in this group (such as more poorly differentiated tumors and more non-luminal A subtypes) (9, 19, 20). The role of molecular subtypes in survival has been proven. Luminal A has the best prognosis, and triple-negative has the worst prognosis (14). The independent effect of the luminal subtype on prognosis was also observed in this study. Multivariate analysis showed that N-stage was not an independent prognostic factor. The same finding was reported in other studies. PIDN is more important than N-stage (21-23). Compared to standard axillary dissection, a lack of the inferiority of sentinel node biopsy was observed in AMAROS (24) and ACOSOG Z0011 trials (25). A sentinel node biopsy was developed in our region about ten years ago. The change in practice did not appear to impair treatment outcomes. There was no significant difference in survival between those who underwent dissection and those with sentinel biopsy.
4.1. Conclusion
The percentage of breast cancer patients under 50 years old is higher in the southeast of Iran than in Western countries. The prevalence of luminal A is lower in the southeast of Iran than in other regions. The survival results were consistent with other studies. The percentage of the involved dissected lymph nodes, group stage, T-stage, M-stage, locoregional recurrence, and luminal subtype were independent prognostic factors for survival.