1. Background
Most patients with chronic kidney disease develop secondary hyperparathyroidism (SHPT), which is identified by elevated levels of parathyroid hormone (PTH) and the consequent progression of the disease. Secondary hyperparathyroidism results from an imbalance in serum calcium and phosphorus levels and an alteration of vitamin D metabolism, which can lead to renal osteodystrophy, pathologic skeletal fractures, cardiovascular disease, and even death (1). The main goal of all SHPT treatments, which have progressed over the past decades, is to maintain normal serum calcium and phosphorus levels. Several extensive observational studies have shown that higher doses of vitamin D are effective at suppressing hyperparathyroidism through the activation of VDR (2).
On the other hand, high doses of vitamin D may exacerbate hyperphosphatemia and hypercalcemia and increase the potential risk of cardiovascular calcification (3). Recently, evidence has shown that a combination of low-dose vitamin D and increased synacle is more effective for treating SHPT (4). Calcitriol is the most known active form of vitamin D3, which affects the parathyroid gland and stimulates calcium transfer in the gut. Adding cinacalcet to calcitriol increases the chances of normalizing PTH levels and allows lower doses of vitamin D analogs that are less likely to cause hypercalcemia and hyperphosphatemia (5, 6).
2. Objectives
Several patients with end-stage renal disease (ESRD) need new therapies with better efficacy and lower side effects. Therefore, this study compared the effectiveness of calcitriol and cinacalcet versus standard-dose calcitriol in treating severe secondary hyperparathyroidism in hemodialysis patients.
3. Methods
3.1. Study Design
This open-label phase II randomized clinical trial was conducted on patients with chronic renal failure and severe secondary hyperparathyroidism referred to Sayad Shirazi and five educational centers in Gorgan, Iran, for hemodialysis. Patients with written informed consent were asked to record their age, sex, body mass index, heart failure, smoking history, and dialysis duration on a pre-designed checklist. The weight and height of patients were accurately measured with a standard scale of the Seca model, and then, the body mass index (BMI) of patients was calculated in kilograms per square meter. The patient’s blood pressure was measured with a sphygmomanometer while sitting. A maximum of 10cc of blood samples was taken for initial tests, including calcium, phosphorus, PTH, and albumin levels.
Then, patients were randomly divided into two intervention groups using the four-block method. Group A received calcitriol 1 mg every other night; group B received calcitriol 1 mg every other night, and cinacalcet 30 mg daily. Randomization was achieved by using dice to select the first block, and patients were selected according to their position in the initial blocks to the end.
Plasma iPTH and serum calcium, phosphorus, and albumin levels were measured three and six months after treatment at Sayad Shirazi Hospital laboratory, Gorgan, Iran.
3.2. Sample Size
This clinical trial study was performed on 70 patients with severe secondary hyperparathyroidism who underwent analysis at the Sayad Shirazi hospitals in Gorgan. The sample size was estimated by randomization with quadruple blocks based on Choulwar et al. (7), considering the 67.86% effectiveness of the cinaclest and 30% calcitriol with a power of 90% (type II error 10%) and type I error 5% (95% confidence factor) and using Equation 1:
3.3. Randomization Method
The project manager used A randomized block method to assign eligible patients between experimental and control groups. Random allocation using blocks of 2 starts by throwing 35 regular hexagons, then generating a sequence of 35 blocks of 2 from the following blocks: AB-BA-BB-AA.
3.3.1. Inclusion Criteria
- Patients at least 18 years of age who underwent dialysis for at least three months.
- iPTH level of over 800 picograms per deciliter (severe secondary hyperparathyroidism).
- Not taking medications such as flecainide, thioridazine, and tricyclic antidepressants.
Lack of liver disease in patients with more than 8.4 mg/dL of calcium.
3.3.2. Exclusion Criteria
- Evidence of malignancy.
- Medications side effects or hypersensitivity reaction.
- History of parathyroidectomy or parathyroid adenoma (confirmed by parathyroid scan).
- Pregnancy, lactating, or women who did not have a contraceptive method.
3.4. Ethical Issues
This study was conducted after obtaining a license from the Ethics Committee of Golestan University of Medical Sciences (registration code: IR.GOUMS.REC.1398.042) and registration in the Iranian Clinical Trials registry (IRCT2020021808046533N1). The informed consent was obtained from all patients, and the researcher kept their information confidential. Participants were free to leave the study at any stage if they were unsatisfied.
The CONSORT reporting guidelines were used and cited as Schulz KF, Altman DG, Moher D, for the CONSORT group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials.
3.5. Objectives and Outcomes, and Measures
Primary outcome: The primary outcome measure was serum iPTH ≤ 300 pg/mL.
Secondary outcome: The study's endpoint was treatment efficacy, defined as a reduction in parathyroid hormone, serum calcium, and phosphorus levels. Corrected calcium was also measured using corrected Ca = measured Ca+ 0.8 (4-Alb).
3.6. Data Analysis
The data were analyzed using SPSS software (version 24). The normality of quantitative variables was checked using the Shapiro-Wilk test. Mean and standard deviation was used to describe quantitative data, and frequency and percentage were used for qualitative data.
T-student and paired t-tests or the non-parametric equivalents such as Mann-Whitney and Wilcoxon signed-rank tests were performed. The chi-square test was used to determine the relationship between qualitative variables in the two intervention groups. The significance level was considered at 0.05.
4. Results
A total of 70 patients with severe secondary hyperparathyroidism underwent analysis in calcitriol (A) or calcitriol and cinacalcet (B) intervention groups.
Since the data were non-normally distributed, the Mann-Whitney test was used to compare quantitative variables (P < 0.05).
The baseline characteristics of these two groups were almost identical (Table 1, P > 0.005).
The serum levels of calcium and PTH at the endpoint were significantly lower than baseline levels in group A. However, phosphorus and albumin levels did not significantly change during this time interval (Table 2). In group B, serum calcium, phosphorus, and PTH levels were significantly lower than the baseline level at the end of the study, but albumin levels did not change significantly during this time interval.
| Characteristics | Calcitriol (n = 35) | Calcitriol + Cinacalcet (n = 35) | P-Value |
|---|---|---|---|
| Age (y) | 54.0 ± 14.5 | 50.8 ± 14.6 | 0.366 |
| Sex (male) | 20 (57.1) | 22 (62.9) | 0.808 |
| BMI (kg/m2) | 24.7 ± 4.9 | 25.2 ± 4.1 | 0.664 |
| Duration of dialysis (m) | 4.97 ± 1.65 | 5.48 ± 4.58 | 0.535 |
| DM (yes) | 7 (20.0) | 9 (25.7) | 0.777 |
| HTN (yes) | 17 (48.6) | 20 (57.1) | 0.632 |
| CHF (yes) | 12 (34.3) | 8 (22.9) | 0.428 |
| Smoking (yes) | 9 (25.7) | 12 (34.3) | 0.603 |
The Comparison of the Distribution of Essential Patient Characteristics in the Two Intervention Groups, A and B a
| Parameter | Baseline | Three Months After the Intervention | End of Study (Six Months After Intervention) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | |
| Ca (mg/dL) | 9.42 ± 0.42 | 9.00 ± 0.35 | 9.07 ± 0.42 | 8.96 ± 0.37 | 8.68 ± 0.73 | 8.77 ± 1.34 | < 0.001 | 0.001 |
| P (mg/dL) | 5.03 ± 0.81 | 6.98 ± 1.36 | 5.06 ± 0.90 | 5.30 ± 1.05 | 5.14 ± 0.74 | 4.11 ± 1.62 | 0.175 | < 0.001 |
| PTH (pg/mL) | 1002.65 ± 182.57 | 1055.42 ± 151.40 | 638.28 ± 146.18 | 474.74 ± 81.28 | 455.68 ± 82.11 | 203.14± 61.57 | < 0.001 | < 0.001 |
| Alb (g/dL) | 4.12 ± 0.97 | 4.74 ± 1.48 | 3.93 ± 0.22 | 3.69 ± 0.28 | 3.87 ± 0.35 | 4.14 ± 0.45 | 0.143 | 0.061 |
The Distribution of Laboratory Parameter Values at Different Time Intervals in Dialysis Patients in Intervention Groups A and B a
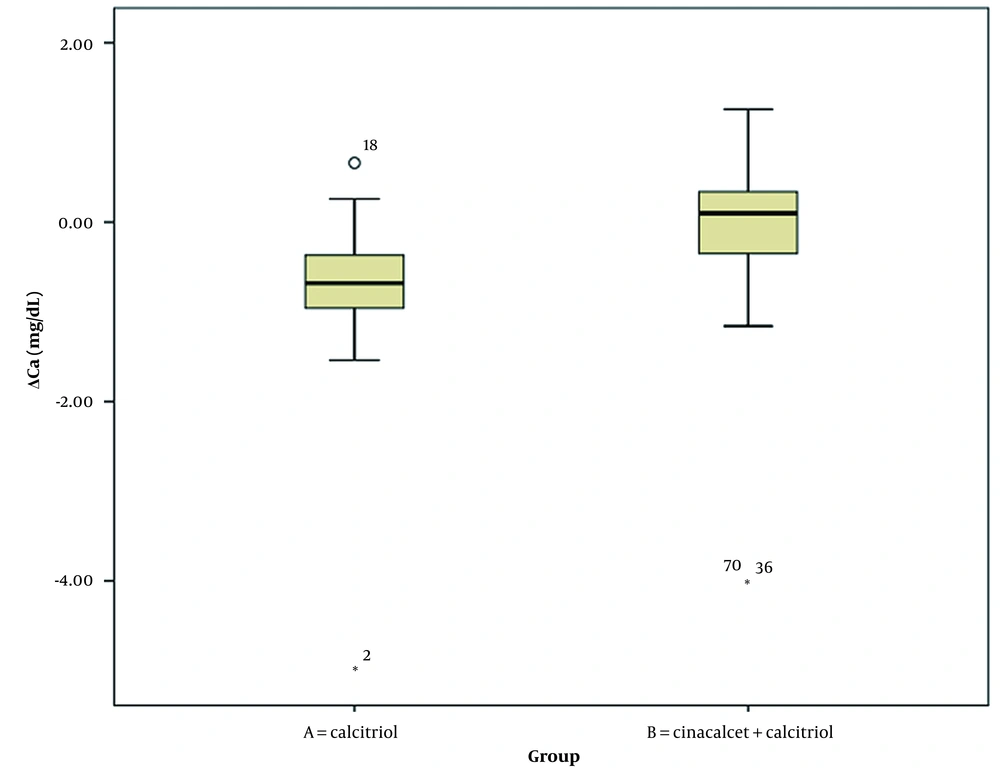
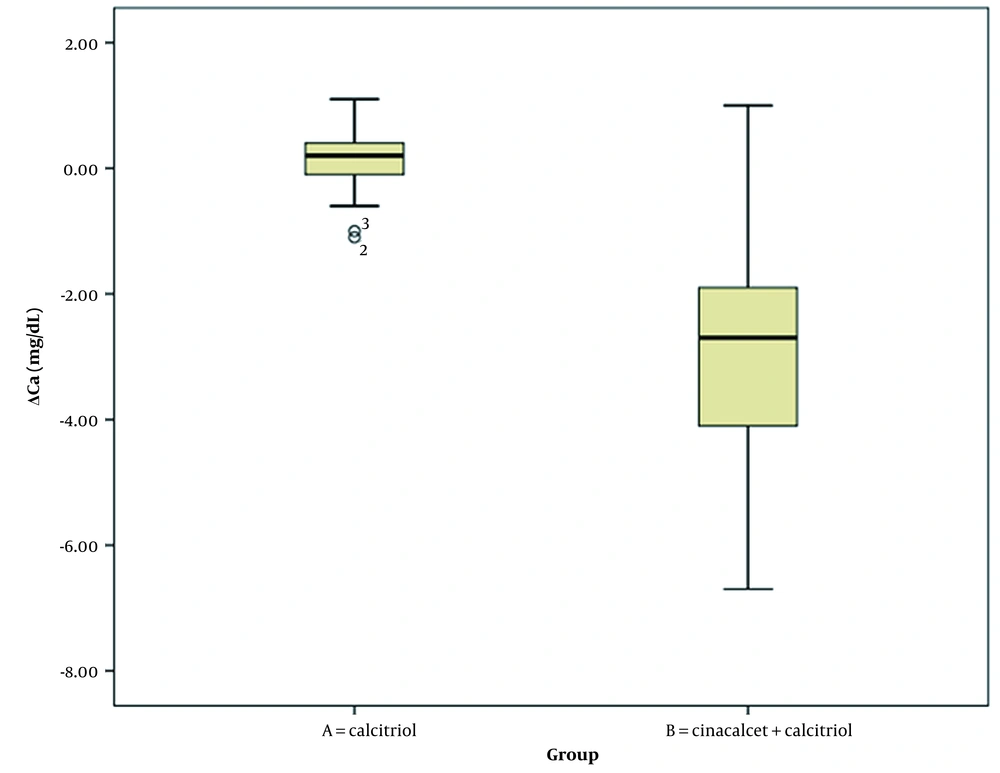
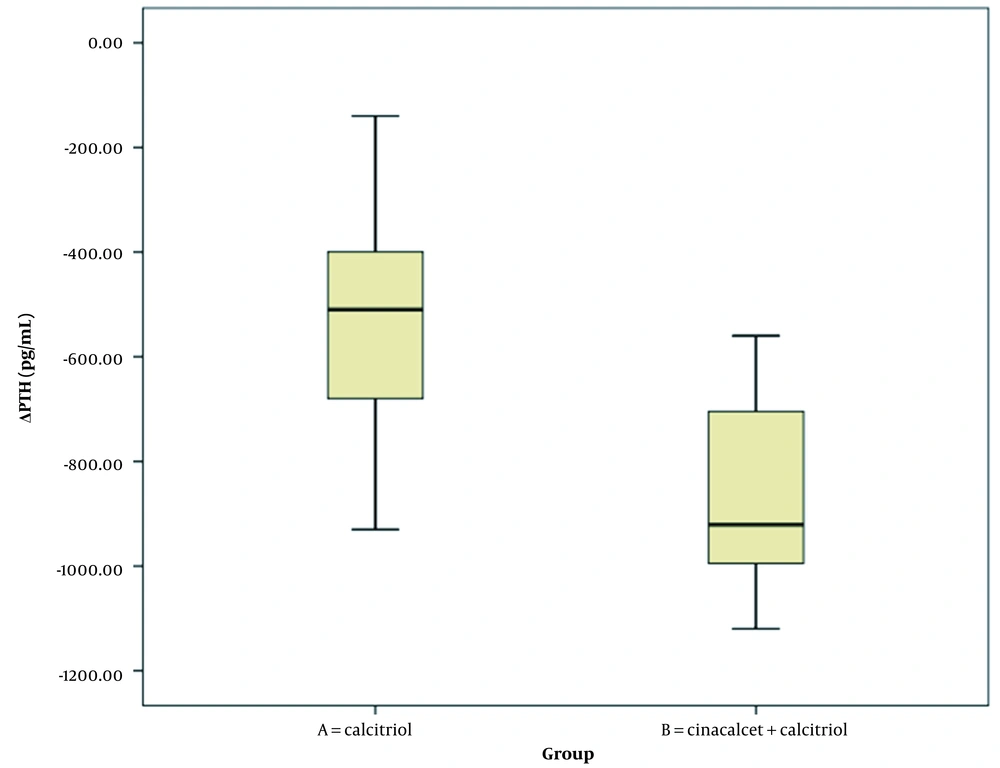
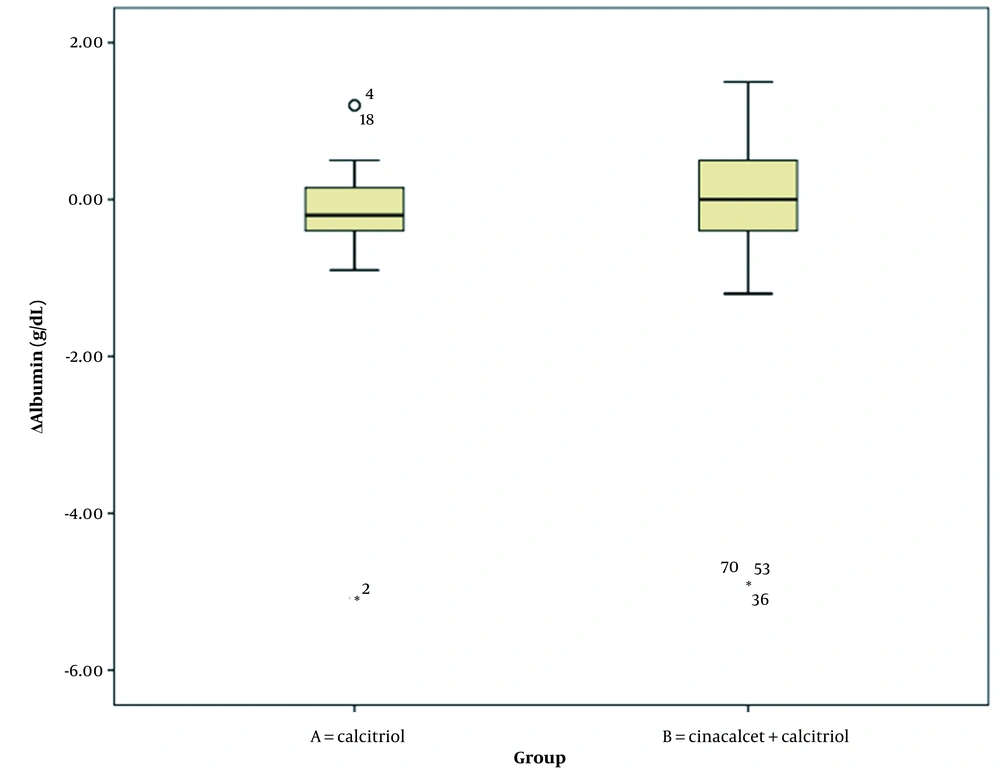
Table 3 summarizes the comparison of changes in laboratory parameters at the beginning and end of the study between the two intervention groups (Wilcoxon signed-rank test). Changes in serum phosphorus and PTH levels significantly differed between intervention groups A and B. However, changes in serum calcium and albumin levels were not significantly different between the two groups (Figures 1, 2, 3, 4 and 5).
| Parameter | Calcitriol (n = 35) | Calcitriol + Cinacalcet (n = 35) | P-Value |
|---|---|---|---|
| ΔCa (mg/dL) | -0.73 ± 0.86 | -0.23 ± 1.31 | 0.062 |
| ΔP (mg/dL) | 0.11 ± 0.47 | -2.87 ± 1.80 | < 0.001 |
| ΔPTH (pg/mL) | -546.97 ± 196.94 | -852.28 ± 172.97 | < 0.001 |
| ΔAlb (g/dL) | 0.24 ± 0.96 | -0.33 ± 1.53 | 0.773 |
Comparison of Changes in the Laboratory Parameters in Dialysis Patients with Severe Secondary Hyperparathyroidism Between Groups A and B a
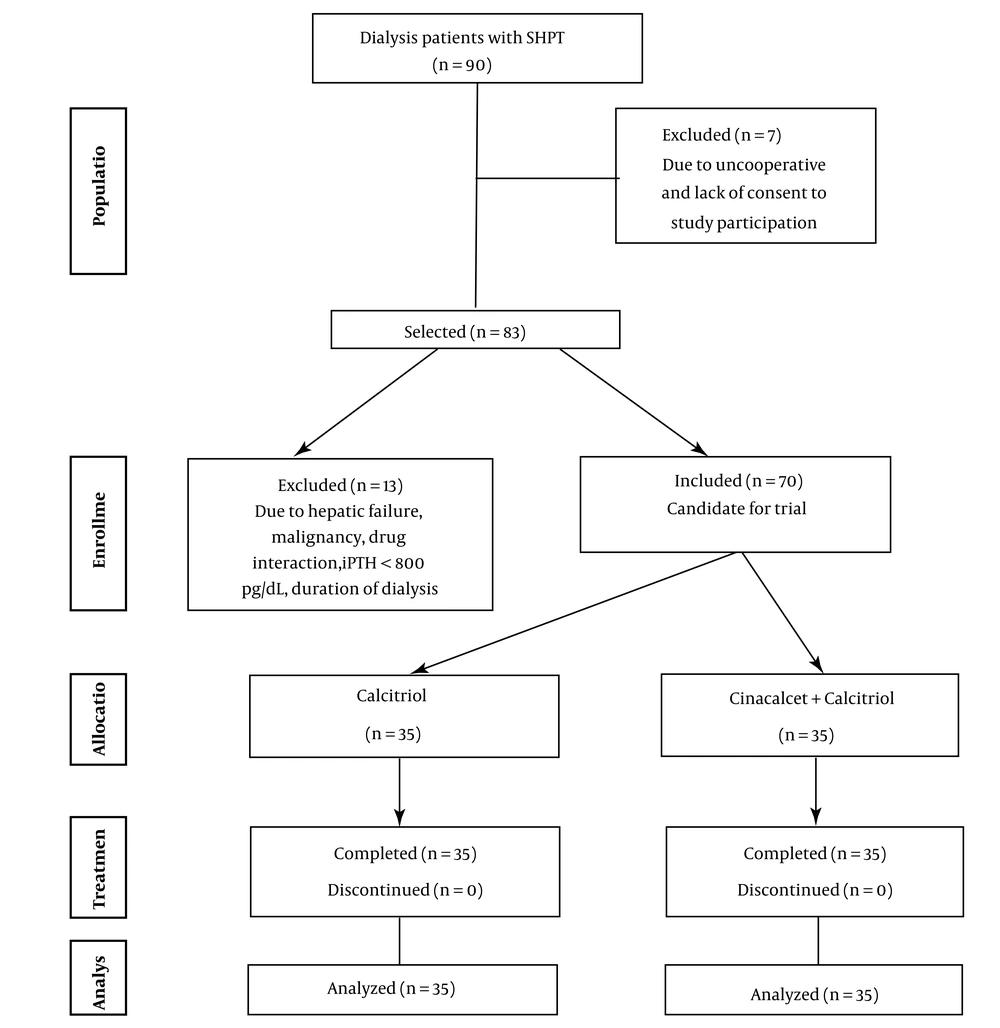
Flow diagram of patient recruitment in a clinical trial (8)
At the end of the study, there were no reports of severe side effects in both groups of patients.
5. Discussion
The present study evaluated and compared the efficacy of two treatment regimens for treating severe SHPT in hemodialysis patients. The results showed that in patients treated with calcitriol alone, serum levels of calcium and PTH were significantly decreased at the end of the study compared to the beginning. However, phosphorus and albumin serum levels did not change significantly during this period. Moreover, serum phosphorus levels were significantly reduced in the group treated with calcitriol, cinacalcet, and calcium and PTH levels. However, the albumin serum level was not significantly reduced. On the other hand, the change rate in serum levels of phosphorus and PTH were significantly different between the two intervention groups, but changes in serum calcium and albumin levels were not significantly different. The role of these factors can be omitted from the results, given that the two groups were similar in age, gender, BMI, duration of dialysis, history of diabetes mellitus, hypertension, heart failure, and smoking.
Secondary hyperparathyroidism is a common, significant, and treatable ESRD complication associated with cardiovascular complications, renal osteodystrophy, vascular calcification, increased bone turnover, fracture risk, and other causes of death in hemodialysis patients. Acidosis, resistance to calcitriol, decreased blood calcium levels due to reduced synthesis of 1,25-dihydroxy vitamin D, and increased serum phosphorus were suggested as causes of elevated serum PTH levels in patients with ESR (9). Since many of the complications of SHPT are irreversible over a long period, timely treatment and early control of its underlying factors are essential (10). Treatment for SHPT in the first stage consists of dietary phosphate restriction, phosphate binders, active vitamin D analogs such as calcitriol, paricalcitol, doxercalciferol, alfacalcidol, oxacalcitriol, falecalcitriol, and finally parathyroidectomy in more severe cases.
In 2004, a calcimimetic called cinacalcet was approved for treating SHPT in dialysis patients (11). The efficacy and safety of cinacalcet have been demonstrated in various studies. Treatment with cinacalcet and active vitamin D in patients with SHPT who were not adequately controlled with standard treatment reduced serum PTH, calcium, and phosphorus levels (9).
Various studies have been performed to compare the effects of different drug regimens, such as cinacalcet and calcitriol, on treating SHPT, which are consistent or inconsistent with the results of the present study. Zawierucha et al. evaluated the consequences of three common SHPT treatment strategies with paricalcitol, cinacalcet, or both drugs over 12 months in all groups, and iPTH levels decreased significantly. However, the level of iPTH was reduced more than in the cinacalcet group in patients undergoing treatment with intravenous paricalcitol and the group treated with concomitant paricalcitol and cinacalcet. A significant change in iPTH level was observed after three months (12). However, combination therapy with cinacalcet and calcitriol was more effective than calcitriol alone and significantly reduced calcium, phosphorus, and PTH serum levels in the present study.
Zheng et al. examined the effects of cholecalciferol in combination with cinacalcet and calcitriol in patients with severe SHPT undergoing hemodialysis. Based on this study, when cinacalcet was combined with calcitriol, cholecalciferol doubled iPTH levels and improved vitamin D levels and femoral neck bone density (8). These results were consistent with the present study’s, and the combined regimen significantly reduced serum iPTH. However, bone density was not assessed in the present study.
Yuan et al. examined the effectiveness of calcitriol combined with cinacalcet on clinical outcomes. The bone metabolism in patients with SHPT undergoing hemodialysis showed that these drugs together could improve high levels of calcium, phosphorus, and iPTH in patients with SHPT and enhance bone metabolism (13), which is in line with the results of the present study. Aggarwal et al. assessed the role of cinacalcet in treating SHPT in CKD and showed that cinacalcet has a unique ability to reduce PTH, calcium, and phosphorus in patients with SHPT (14), consistent with the present study. Khalaf Al-Hwiesh and Abdul-Rahman studied the effects of intermittent cinacalcet doses on treating SHPT in hemodialysis patients and found no significant difference between the two methods in controlling PTH secretion. Although serum phosphorus levels increased significantly at the end of the study, calcium levels did not change in the subjects (15). In the present study, cinacalcet and calcitriol reduced serum PTH, calcium, and phosphorus levels without severe side effects.
5.1. Limitations
Since dialysis patients suffer from many physical problems, their mental-psychological condition is not suitable, which causes their lack of proper cooperation in conducting the study. In addition, the lack of satisfaction of some patients leads to the dropping of samples.
The objectives and benefits of the study were explained to the participants, and the time of data collection and sampling was also increased to solve the above limitations.
5.2. Conclusions
This study showed that concomitant treatment with cinacalcet and calcitriol had a better effect than calcitriol alone and significantly reduced serum calcium, phosphorus, and PTH levels. No severe side effects were reported following these medications. However, more studies are needed in the future to prove these findings.