1. Background
Amniotic fluid surrounds the fetus during development and serves a variety of functions, such as safeguarding the fetus when the mother's abdomen sustains damage by providing a cushion between the fetus and the umbilical cord. Amniotic fluid also safeguards the umbilical cord and lessens the possibility of uterine wall compression (1). Amniotic fluid's antimicrobial properties aid in protecting the fetus from pathogenic pathogens. Furthermore, fluid and nutrition are stored for the fetus to hold the proteins, electrolytes, immunoglobulins, and vitamins. This fluid supplies the environment and essential growth factors for the fetal organs, including the musculoskeletal, digestive, and pulmonary systems, normal growth, and maturation. Doctors can use amniotic fluid to track a pregnancy’s development and forecast fetal outcomes (2).
A woman's amniotic sac is determined by how much fluid enters and leaves. Late pregnancy, swallowing, fetal urination, and lung fluid expulsion significantly contribute to fluid transport, with little help from other sources (3, 4). The amniotic fluid volume changes due to fetal abnormalities that affect any of these functions. As an illustration, growth-restricted fetuses might redistribute blood flow away from their kidneys, which lowers fetal urine production and causes oligohydramnios (5).
A lack of amniotic fluid, or oligohydramnios, can negatively affect the pregnancy's prognosis because amniotic fluid is crucial for maintaining fetal health (6). Low amniotic fluid volume is linked to a higher fetal and neonatal mortality rate and morbidity (7). Many studies have recorded neonatal intensive care unit (NICU) hospitalization, cesarean sections owing to fetal distress, meconium aspiration syndrome, fetal growth limitation, premature birth, and even fetal mortality in pregnancies with oligohydramnios (8-10).
Oligohydramnios is described as an amniotic fluid index (AFI) of 5 cm or less (9), while borderline (marginal) oligohydramnios is defined as an AFI of 5.1 to 8.0 cm (11, 12). In a study on 89050 healthy pregnancies with a singular and non-anomalous fetus at term, oligohydramnios (identified as AFI ≤ 5 cm) was found in 4.4% of the cases (13).
Utero-placental perfusion is increased by sildenafil citrate, a phosphodiesterase type 5 inhibitor (14). Increased uterine blood flow and amplified estrogen-induced vasodilation are the effects of sildenafil citrate (15). Two comprehensive clinical evaluations examined whether sildenafil citrate is safe to use while pregnant. Both trials found that minor maternal side effects, such as headaches, flushing, vision disturbances, dizziness, palpitations, arthralgia, dyspepsia, and epigastric discomfort, were not more common than with placebo (16, 17).
Studying sildenafil citrate is necessary to determine its safety and effectiveness in treating oligohydramnios. In contrast, previous clinical evaluations have found that sildenafil citrate is generally safe for pregnant women and may increase uterine blood flow. Hence, further research is needed to fully understand its potential benefits and risks in treating oligohydramnios. A well-designed study could provide more definitive evidence on the safety and effectiveness of sildenafil citrate for this purpose.
2. Objectives
The current study aimed to evaluate sildenafil's effect on the volume of amniotic fluid in women who had oligohydramnios or borderline oligohydramnios.
3. Methods
3.1. Study Design
Pregnant women diagnosed with oligohydramnios or borderline oligohydramnios participated in this research experiment at Fatemieh Training Hospital in Hamedan, Iran. Women with a singleton pregnancy and gestational age between 24 and 36 weeks diagnosed with borderline oligohydramnios or oligohydramnios (average amniotic fluid index less than 8 cm, determined by transabdominal ultrasound) were included. Patients with preeclampsia, treated with anti-hypertensive drugs, or allergic to sildenafil citrate were excluded. Informed consent was obtained from each participant before enrolling in the study, and patients were included in the study only if they met the eligibility criteria and consented to participate in the clinical trial. The trial was documented in the Iran Registry of Clinical Trials (IRCT) (IRCT20220903055868N1). The study was approved by the Hamedan University of Medical Sciences Ethics Committee (IR.UMSHA.REC.1401.361). The hiring process began in October 2022.
According to this study, 42 women hospitalized in Fatemieh Hospital were divided into two groups. Group A was assigned to the case group, and Group B was related to the placebo group. Block randomization and classification were used to ensure the equality of the two groups in terms of the number and variables affecting the study results. Both groups were the same in terms of maternal age and gestational age. This study is double-blind, so the researcher and the subject needed to be made aware of the allocation status of the groups. Since the drug is prescribed together with other medications. Therefore, the issue is unaware of the status of allocation to the groups, and the researcher who measures the clinical and diagnostic measurements was unaware of the individuals’ status. A supervisor is mindful of the allocation of groups. Sealed and hidden envelopes were used during random distribution. Additionally, a statistician unaware of the study's groups conducted the study's statistical analysis.
The primary amniotic fluid index of the patients was measured when hospitalized. In the intervention group, 3 liters of isotonic solution (Ringer lactate) were administered intravenously every 24 hours with 50 mg of oral sildenafil citrate (Pharma Chemistry-Iran) every 8 hours and hydration treatment, and a placebo was given to the control group. Betamethasone injections were given to women whose gestational age was less than 36 + 6 weeks. The amniotic fluid index was remeasured one and two weeks after the intervention. The woman was released if an ultrasound revealed a rise in the amniotic liquid index of at least 8 cm.
3.2. Data Collection Tools
A nonstress test (NST) was used to check the fetus’s health twice a week. At the same time, biophysical profiles and measurements of the amniotic fluid volume by abdominal ultrasonography were performed once a week.
The frequency of maternal pregnancy termination and side effects of the drug were investigated and compared.
3.3. Statistical Analysis
Statistical analysis of the data was performed using SPSS version 23. The significance threshold was deemed to be 0.05 or lower. The mean and standard deviation were used to describe quantitative data, whereas frequency and percentages were used to display qualitative data. The t-test or Mann-Whitney test was used to analyze quantitative variables between the two groups statistically. In contrast, the chi-square or Fisher test was used for the statistical analysis of qualitative data. The Friedman test was used to compare the amniotic index three times. A 95% confidence interval odds ratio and the logistic regression test were performed to assess the effects.
4. Results
A total of 42 women participated in this study; 3 in the intervention group and 5 in the control group were terminated during the survey. As shown in Table 1, maternal age, gestational age, and amniotic fluid index before the intervention are not significantly different between the two groups (P-value > 0.05).
| Sildenafil | Placebo | P-Value a | |||
|---|---|---|---|---|---|
| Count | Mean ± SD | Count | Mean ± SD | ||
| Age | 21 | 25.24 ± 6.69 | 21 | 26.60 ± 7.50 | 0.539 |
| Gestational age | 21 | 30.43 ± 2.46 | 21 | 31.00 ± 2.43 | 0.453 |
| Basic amniotic fluid | 21 | 5.24 ± 1.18 | 16 | 5.52 ± 1.03 | 0.413 |
Abbreviation: SD, standard deviation.
a Independent t-test.
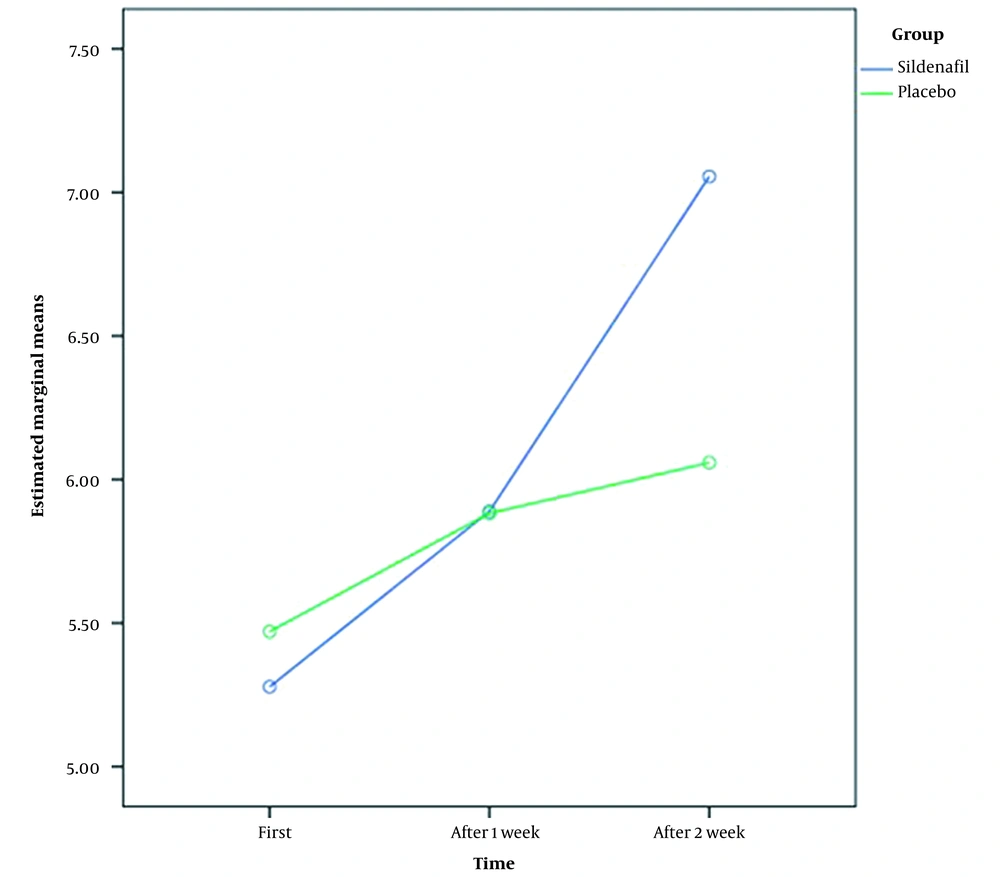
Average indices were compared in two groups three times (at the primary, after 1st week, and 2nd week after treatment). Additionally, the amount of increased amniotic fluid in each group was examined.
According to Table 2, the amniotic fluid was not significantly different between the two groups during the primary, after 1st week, and 2nd week after treatment (P-value > 0.05). However, there was a considerable variation in the study group's amniotic fluid levels from primary to two weeks after treatment (P-value < 0.05). As considered in Table 2, the amniotic fluid levels were increased over time in two groups.
Abbreviations: AFI, amniotic fluid index; SD, standard deviation.
a Mann-Whitney.
b Independent t-test.
c Friedman test.
During the first and second weeks, the amniotic fluid change was compared between the two groups. Over one week, neither group's amniotic fluid index increases significantly (all P-values > 0.05). This change was significant at the end of two weeks. A significant difference was noted between the amniotic fluid index of the intervention group and its primary index during hospitalization compared to the control group (Table 3 and Figure 1).
| AFI | Sildenafil | Placebo | P-Value a | ||
|---|---|---|---|---|---|
| Count | Mean ± SD | Count | Mean ± SD | ||
| After 1nd week- basic | 21 | 0.57 ± 0.93 | 21 | 0.38 ± 1.47 | 0.630 |
| After 2nd week- basic | 18 | 1.78 ± 1.86 | 16 | 0.59 ± 1.33 | 0.048 |
Abbreviations: AFI, amniotic fluid index; SD, standard deviation.
a Independent t-test.
There was no significant difference between the two groups regarding pharmacological side effects (all P-value > 0.05) (Table 4).
5. Discussion
The results demonstrated that sildenafil citrate and hydration, compared to hydration alone, can improve AFI without causing negative side effects and is an effective treatment option. Currently, various techniques are used to treat and control oligohydramnios. Amnioinfusion is an invasive method with potential risks of increasing the amount of amniotic fluid (18). Maternal hydration techniques can also improve amniotic fluid, but it is not yet clear how AFI changes following maternal hydration and how long these changes last (19, 20). The oral sildenafil administration method is non-invasive and safer, resulting in fewer complications and more patient comfort.
Sildenafil was shown to be effective at reducing AFI in our research, but we did not investigate the exact mechanism by which it may have such an effect. Vasodilation was proposed to be brought on by sildenafil, which may enhance uteroplacental perfusion, raising fetal renal blood flow, fetal urine output, and AFI (21). This increase in AFI improved pregnancy and neonatal outcomes.
The results of this study are consistent with those of Choudhary et al.’s study, which reported a significant increase in the amniotic fluid index following the use of sildenafil citrate during pregnancy, and no specific side effects for the mother or fetus were reported (22). In addition, Maher et al. showed that administration of sildenafil citrate and intravenous hydration is associated with greater amniotic fluid volume with more prolonged pregnancy compared to hydration alone (23). Nath et al. indicated that patients with oligohydramnios could significantly improve with sildenafil citrate (24). In line with Dunn et al., in the current study, women who used sildenafil citrate did not experience any significant negative effects and confirmed the safety of this treatment (16).
The following are some of the positive aspects of the research: (1) an appropriate randomization procedure was used; (2) a power analysis was conducted; (3) continuous or temporary increases in AFI were measured, and the mother and fetus were continuously monitored during the study; (4) the participants' gestational ages ranged from 24 to 36 weeks, which represents a considerable variation; (5) the clinical significance of improving AFI was evaluated; and (6) the control group was given a placebo.
Nevertheless, some negative aspects remain, including the following: (1) despite our best efforts, the results should be interpreted cautiously because measuring AFI is subjective and challenging due to fetal movement. There is a poor correlation between ultrasonography and actual amniotic fluid volume. Hence, such obstacles were overcome by selecting an ultrasonographer blinded to the treatment arms; (2) pregnancies that were considered normal before 24 weeks of gestation and pregnancies and complicated for either the mother or the fetus were excluded; (3) since the mother's posture was not standardized throughout hospital treatment, its effect on changes in AFI rates is unclear; (4) features of amniotic fluid, such as the solute contents after therapy, were not investigated. In contrast to real amniotic fluid volume, AFI parameters were used to measure improvement after treatment; (5) the circulating plasma volume rises during pregnancy, which may modify the pharmacokinetics of the medication. However, data on pregnancy are restricted, so it is difficult to determine an appropriate dose of sildenafil or a dosage and administration schedule. Taking 100 mg of sildenafil orally results in a plasma concentration of more than 100nmol/L for 4 - 5 hours, which is 100 times lower than the concentration needed for nonspecific inhibition of other phosphodiesterase (PDE) isoforms.
5.1. Conclusions
Based on the results, sildenafil citrate consistently increased AFI without causing negative embryonic consequences. Future studies should consider more samples, the outcome of the newborn, including the Apgar score of the newborn and the hospitalization rate of the newborn in the NICU.