1. Background
Helicobacter pylori (H. pylori) is a gram-negative, flagellated, microaerophilic bacterium that colonizes the human gastric mucosa (1) and causes chronic gastritis, peptic ulcer, and gastric cancer, as well as mucosa-associated lymphoid tissue (MALT) lymphoma. Recent studies have reported that H. pylori infection is an independent risk factor associated with unexplained iron-deficiency anemia and micronutrient deficiency (iron and vitamin B12) (2, 3).
H. pylori infection is a severe public health problem infecting nearly 50% of the world’s population (4). The prevalence of H. pylori infection in Iran has been reported from 36% to 90% in different provinces (5). Managing H. pylori-related complications requires H. pylori eradication. The recommended treatment regimen for H. pylori infection is triple therapy consisting of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin. Quadruple therapy, sequential therapy, and triple therapy using new antimicrobials, such as levofloxacin, rifabutin, and furazolidone, might be triple therapy alternatives (6). Furthermore, the issues of indigenous gut microbiota and antimicrobial-resistant bacteria can be influenced by antibiotic treatment. The gut microbiota can positively impact health, and an imbalance in the microbiota is associated with many disease states. The high rate of antibiotic resistance to various prescribed therapy regimens is an essential factor in the failure of H. pylori treatment (7).
H. pylori poses significant health risks in Iran due to the low eradication rate and a re-infection rate of about 20% (8). Recent research has suggested that certain probiotic species, such as Lactobacillus, Bifidobacterium, and Saccharomyces boulardii, may benefit H. pylori in vitro and in vivo (9, 10). Some studies have even indicated that taking probiotics alongside eradication treatment may improve the success rate of H. pylori removal and relieve gastrointestinal symptoms (11, 12).
2. Objectives
However, the validity of these findings is still disputed. Therefore, this clinical trial aimed to assess the role of Saccharomyces boulardii (S. boulardii) in combination with a quadruple regimen for H. pylori eradication and the associated antibiotic side effects.
3. Methods
3.1. Study Design and Patients
This double-blind, randomized, placebo-controlled study was conducted on adult patients aged 18 - 65 years with H. pylori infection who were referred to the Gastroenterology Clinic of the Imam Reza Therapeutic Educational Hospital (Kermanshah, Iran) between January and October 2022. H. pylori infection was confirmed by a positive urea breathing test (UBT) or positivity in both the rapid urease test (RUT) and histology for patients undergoing gastrointestinal endoscopy. The exclusion criteria included chronic diseases such as inflammatory bowel, celiac, and renal failure, malignancies, antibiotics and/or proton-pump inhibitors and/or H2 receptor blockers therapy within the previous one-month, non-steroidal anti-inflammatory drug use; alcohol abuse, drug addiction, and long-term use of corticosteroids.
3.2. Ethics
The research protocol was approved by the ethics committee of Kermanshah University of Medical Sciences, Kermanshah, Iran (IR.KUMS.MED.REC.1401.122). The registration ID of the study in the Iranian Registry of Clinical Trials was IRCT20130812014333N191. A written informed consent was obtained from all subjects before they participated in the study.
3.3. Randomization and Allocation
Random assignment of volunteers in a 1: 1 ratio was conducted using a simple random allocation procedure with Excel. The 142 eligible enrolled volunteers were divided into two groups of 71. The assigned group for each patient was determined by selecting a sealed envelope numbered from one to 142.
3.4. Study Procedure
The probiotic supplementation group received a quadruple eradication therapy regimen including a dose of 40 mg of pantoprazole twice daily, bismuth subcitrate, 120 mg four times daily, two antibiotics (amoxicillin, 500 mg twice daily, and metronidazole, 500 mg three times daily, for ten days) plus probiotic supplementation, twice daily for four weeks. The probiotic supplementation (Daily East) contained 250 mg of living S. boulardii (10 × 109 CFU). The placebo group received the same quadruple eradication therapy regimen and a placebo twice daily for four weeks. The placebo capsules contained the same substance without yeast and were packed in identical capsules, coded by the producer to ensure guarantee blinding. The probiotic supplement and placebo capsules were produced by ZistTakhmir Pharmaceutical Company, Tehran, Iran.
3.5. Clinical and Laboratory Monitoring
H. pylori infection was assessed using a stool antigen test for H. pylori (Helicobacter pylori Stool Antigen ELISA Kit, Pishtaz Teb Co, Iran) six weeks after the completion of treatment. The success of eradication therapy was determined by a negative stool antigen test for H. pylori infection.
3.6. Outcomes
The primary outcome was H. pylori eradication at six weeks after randomization. Side effects were assessed in participants who began their assigned dietary supplements and treatment. Therapy-related side effects were collected through self-reports.
3.7. Statistical Analysis
The SPSS software version 20.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. Categorical and continuous variables are frequency (percentage, %) and mean ± standard deviation (SD). Categorical variables were compared using the chi-square test. Independent-samples t-test or Mann–Whitney U test was used to compare the means of continuous variables as appropriate. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Patients
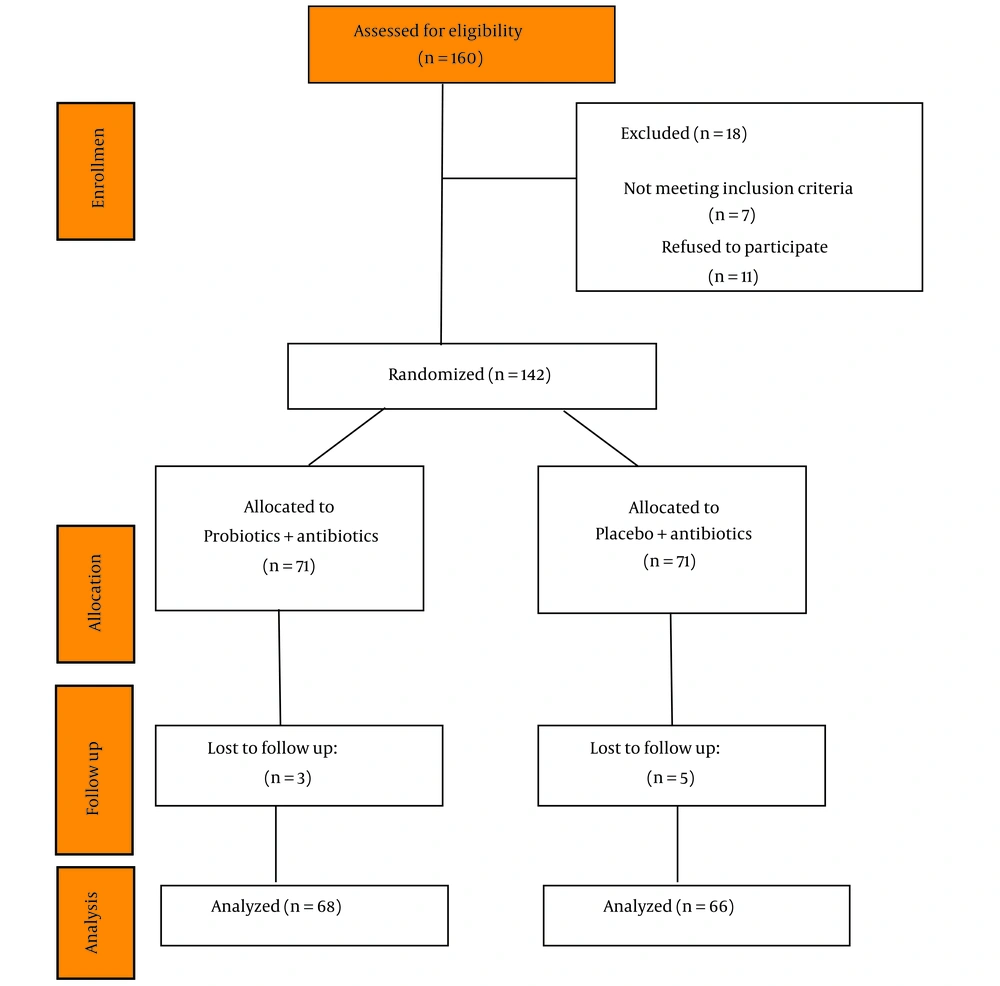
Among the 160 patients screened, 18 were excluded for various reasons (Figure 1). A total of 142 patients were randomized in this study (31.6% male; mean age 45.29 years). Endoscopic analysis revealed erythema antrum in 36.6% of patients, erythema duodenum in 12.6%, and macroscopic nodular antral gastritis with hyperemia in 29.6%. There were no significant differences in the baseline characteristics of patients included in the two study groups (Table 1). A total of 134 H. pylori-positive patients (94%) completed therapy, while three and five subjects were withdrawn from probiotics and placebo groups, respectively.
| Variables | Probiotics Group (N = 71) | Placebo Group (N = 71) | P-Value |
|---|---|---|---|
| Age (y) | 46.07 ± 12.25 | 44.51 ± 11.63 | 0.45 b |
| Gender, male | 21 (29.6) | 24 (33.8) | 0.58 c |
| Endoscopic findings | |||
| Erythema antrum | 30 (42.3) | 22 (31) | 0.16 c |
| Erythema duodenum | 6 (8.5) | 12 (16.9) | 0.13 c |
| Macroscopic nodular antral gastritis with hyperemia | 21 (29.6) | 21 (29.6) | - |
| Normal | 10 (14.1) | 11 (15.5) | 0.81 c |
a Values are presented as No. (%) or mean ± SD.
b An independent sample t-test.
c Chi-square test.
4.2. Helicobacter Pylori Eradication Rate
No significant differences between the study groups were observed in H pylori eradication rates (P = 0.27). Treatment was successful in 30 of 68 (44.1%) subjects in the probiotic group compared with 23 of 66 patients in the placebo group (34.8%).
4.3. Safety
There was significantly a lower rate of nausea (P = 0.04), taste disturbance (P = 0.002), headache/dizziness (P = 0.002), flatulence/epigastric pain (P = 0.04) in the probiotic group compared with the placebo during the treatment. There was no significant difference between the experimental and control groups regarding vomiting, diarrhea or constipation, and heartburn (Table 2).
| Outcome | Probiotics Group (N = 68) | Placebo Group (N = 66) | P-Value |
|---|---|---|---|
| Eradication at least six weeks after randomization | |||
| Positive | 30 (44.1) | 23 (34.8) | 0.27 |
| Negative | 38 (55.9) | 43 (65.2) | |
| Side effects | |||
| Nausea | 4 (5.9) | 11 (16.7) | 0.04 |
| Vomiting | 1 (1.5) | 4 (6.1) | 0.16 |
| Diarrhea | 0 | 2 (3) | 0.14 |
| Constipation | 0 | 0 | - |
| Heartburn | 5 (7.4) | 12 (18.2) | 0.06 |
| Taste disturbance | 3 (4.4) | 15 (22.7) | 0.002 |
| Headache/dizziness | 1 (1.5) | 11 (16.7) | 0.002 |
| Flatulence/epigastric pain | 1 (1.5) | 6 (9.1) | 0.04 |
a P-values calculated by Chi-square test.
5. Discussion
Probiotics were associated with an approximate 10% increase in the eradication rate of H. pylori and a reduction in the incidence of nausea, taste disturbance, headache/dizziness, flatulence/epigastric pain in the probiotic supplementation group compared to the placebo group.
The primary cause of adverse effects during anti-H. pylori therapy uses moderate to high doses or combination antibiotics (13). Antibiotic-related side effects are often experienced and typically impact the digestive system. The colon can contain up to 1014 CFU/mL of bacteria that comprise the intestinal microbiota. The target microorganisms and the gut commensal microbiota coexist in a delicate balance easily disturbed by antibiotic therapy, leading to the emergence of potentially pathogenic species over saprophytic flora. The three antibiotics (amoxicillin, clarithromycin, and metronidazole) that are most commonly used in H. pylori therapy were associated with gastrointestinal complications (14). Clarithromycin, in particular, increased the smooth muscle of the gastrointestinal tract, which accelerated transit time and led to diarrhea (13).
Several studies have evaluated the effect of probiotics on Helicobacter Pylori Eradication and found them to be effective supportive therapy. Oh et al. assessed the influence of probiotic supplementation on gut microbiota during H. pylori eradication using high-throughput sequencing (15). In this study, probiotic supplementation could reduce the fluctuation of gut microbiota, improving the success rate of H. pylori eradication by limiting the growth of antibiotic-resistant bacteria. Furthermore, Dang et al. conducted a meta-analysis of supplemental probiotics in eradication therapy and side effects during eradication therapy (16). Among 33 randomized trials, the H. pylori eradication rate in probiotics supplementation groups was significantly higher than in controls. Furthermore, there was a significant correlation between groups in improving discomfort symptoms. Probiotics may be effective against H. pylori infection due to stabilizing or restoring endogenous physiological flora and preventing H. pylori growth (17).
In comparison to quadruple therapy alone, quadruple therapy plus probiotics can significantly reduce side effects, including nausea, bitter taste, headache/dizziness, and flatulence/epigastric pain. A clinical trial by Seddik et al. showed that the combination of sequential therapy and S. boulardii significantly reduced the incidence of antibiotic-associated diarrhea compared to sequential therapy alone (18). Another study reported that supplementation of non-viable L. reuteri with triple therapy reduced the frequencies of abdominal distention, diarrhea, and the GSRS score (19).
Several mechanisms have been proposed to clarify the effect of probiotics on H. pylori growth, including (1) preventing H. pylori colonization by defeating gastric epithelial receptors or co-aggregation mechanisms; (2) producing bacteriocins, organic acids, and bio-surfactants that are anti-H. pylori; (3) supporting intestinal tissues by enhancing mucin synthesis; (4) modulation of immune system response; (5) inducing antigen-specific antibodies; and (6) decreasing gastric mucosal inflammation (10, 14, 20).
5.1. Conclusions
The eradication rate for H. pylori infection was not significantly different between the placebo and probiotic groups, but it improved by approximately 10% in the probiotic group. Furthermore, the probiotic group reduced the side effects caused by the treatment. Further studies with different probiotic combinations or treatment duration are recommended to enhance the eradication of H. pylori infection.