1. Background
Gestational diabetes mellitus (GDM) is called glucose intolerance, which is diagnosed during pregnancy (1-3) and is considered the most common metabolic disorder during pregnancy (1). The outcome, complication occurrence rate, and severity depend on onset time, glucose intolerance duration in pregnancy, maternal diabetes severity, and control level (4). The GDM prevalence increases with maternal age, racial/ethnic changes in childbirth, and obesity (5).
Based on different screening methods and diagnostic and population criteria, gestational diabetes is between 1 - 14% and has an average prevalence of 7.5% (4, 6). However, some cases include undiagnosed diabetes before pregnancy (7). According to various studies, the prevalence of gestational diabetes in Iran is 1.4 to 8.9% (8, 9).
Perinatal complications associated with GDM include high blood pressure disorders and the risk of preeclampsia, premature birth, shoulder dystocia, stillbirth, neonatal hypoglycemia and hypocalcemia, hyperbilirubinemia, cesarean delivery, birth injuries caused by fetal macrosomia, polyhydramnios, and higher prevalence of bacterial and fungus infections. Postpartum complications lead to obesity, impaired glucose tolerance in children, and diabetes and cardiovascular diseases in mothers. Continuous control of maternal blood sugar, examination of the fetus for fetal distress, and fetal weight monitoring through ultrasound, maternal weight management, nutritional therapy, physical activity, and medication can reduce the complications associated with GDM (7, 10, 11).
Mother’s high blood sugar during pregnancy leads to an increase in insulin and subsequently increases the fat cell production in the fetus, which increases the possibility of obesity and insulin resistance in childhood and diabetes in adulthood (7, 11). The complications are also reduced with timely diagnosis and treatment of gestational diabetes. Therefore, the cases of macrosomia, shoulder dystocia, and cesarean delivery are reduced by 50% (7, 11). Currently, the best way to screen for gestational diabetes is to perform a blood sugar test after consuming 50g of oral glucose, which is called a glucose challenge test (GCT) (7).
These tests are performed at 24 - 28 weeks of pregnancy. However, women who have risk factors such as obesity, a history of macrosomic birth, abnormalities in previous babies, unexplained perinatal death in previous pregnancies, and a family history of diabetes in a first-degree relative may be requested at the first perinatal visit to perform these tests. The appropriate base point for considering the glucose challenge test abnormal is one of the critical issues discussed in gestational diabetes screening (7, 11).
In general, if the GCT level ≥ 140 mg/dL, 80% of gestational diabetes cases can be identified, and 14 - 18% of cases can be false positives, and if the standard is ≥ 130 mg/dL, more than 90% of cases of gestational diabetes are diagnosed, but 20 - 25% of cases can be false positive (7).
Fetal heart rate (FHR) ultrasound recording is essential to routine care during pregnancy and delivery. There is no doubt that FHR is a critical indicator of fetal outcomes (12). Various studies have been conducted on the effects of gestational diabetes on fetuses and mothers and its consequences. In addition, several studies have been conducted on the impact of gestational diabetes on fetal heart rate (12-14).
As gestational diabetes poses risks to the mother and fetus, it is crucial to identify and control it as soon as possible.
2. Objectives
The present study was conducted to investigate the relationship between fetal heart rate in the first trimester and gestational diabetes. The positive results of this study can prevent gestational diabetes risks for the mother and fetus.
3. Methods
The present cohort study was started after the approval and adoption of the ethics code (IR.KUMS.REC.1399.586). In the first trimester (11 - 14 weeks), pregnant women referred to the ultrasound department of Imam Reza Hospital (Iran) for nuchal translucency (NT) sonography were studied.
The inclusion criteria were singleton pregnancy and consent to enter the study, and exclusion criteria included the presence of congenital abnormalities in the latest or previous pregnancies, diabetes Mellitus or history of gestational diabetes, abnormal screening test, and NT > 95th centile, medication and smoking by the mother, and maternal thyroid disorders. The sample size was 96 mothers in each group, and a total of 192 mothers were required for the study according to the reference study (14) and average FHR in fetuses of mothers with gestational diabetes (145.3 ± 10.6) and healthy (141.15 ± 10.2) with 95% confidence and 80% power test.
Informed consent was obtained from the mothers before they were included in the study. Then, the heart rate of the fetuses was checked at the time of NT measurement using the Supersonic Mac30 ultrasound machine and the Convex 5 MHz probe, and the findings were recorded along with the mother’s demographic information. Fetal heart rate was evaluated in the transverse section of the fetal chest and at the level of the tricuspid valve. Then, after performing the sugar tolerance test (at 24 - 28 weeks of pregnancy) and determining the result of gestational diabetes, the mothers were divided into two groups (healthy and those with gestational diabetes). Then the fetuses FHR was again measured by ultrasound. Recent sonographic findings were added to the previous data collection form, including FHR, fetal age, and maternal glucose tolerance test results.
The data were analyzed by SPSS software version 16. Quantitative data such as maternal height, weight, age, and fetal heart rate were examined using tables, graphs, frequency of data, mean, and standard deviation. The Kolomogrov-Smirnov test was used to check the normality of quantitative variables. Based on the Kolomogrov-Smirnov test, an independent t-test was utilized to analyze normal data, and Mann-Whitney U test was used for non-normal data. Along with the above tests, the chi-square test was used to determine the homogeneity of the groups. ROC curve and calculation of RR and R2 were used to predict the probability of developing gestational diabetes. A significance level of 0.05 was considered for all the tests.
4. Results
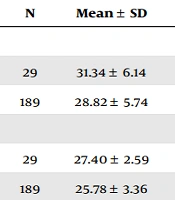
Initially, 300 pregnant women were included in the study, but 86 were excluded for reasons such as abortion or non-cooperation. Finally, the study was conducted on 214 pregnant women (including 29 mothers with gestational diabetes and 185 healthy mothers). The heart rate of the mothers’ fetuses was measured once in the first trimester (11 - 14 weeks) and once after the glucose tolerance test. Maternal age, BMI, and fetal heart rate in the first trimester and after the glucose tolerance test were investigated among mothers with gestational diabetes and healthy mothers (Table 1).
| Variables and Gestational Diabetes | N | Mean ± SD | P-Value |
|---|---|---|---|
| Mother’s age | 0.030 | ||
| Yes | 29 | 31.34 ± 6.14 | |
| No | 189 | 28.82 ± 5.74 | |
| Mother’s body mass index | 0.014 | ||
| Yes | 29 | 27.40 ± 2.59 | |
| No | 189 | 25.78 ± 3.36 | |
| FHR in the 1st trimester | 0.930 | ||
| Yes | 29 | 164.38 ± 7.20 | |
| No | 189 | 164.25 ± 7.43 | |
| FHR after the glucose tolerance test | 0.326 | ||
| Yes | 29 | 144.97 ± 7.60 | |
| No | 189 | 146.65 ± 8.71 |
Abbreviation: FHR, fetal heart rate.
The average maternal age (P = 0.030) and body mass index (P = 0.014) in mothers with gestational diabetes significantly differed from healthy mothers. However, there was no significant difference in fetal heart rate in the first trimester (P = 0.930) and after the glucose tolerance test in mothers with gestational diabetes and healthy mothers (P = 0.326).
In addition, the mean rank of CRL and NT in the fetuses of mothers with gestational diabetes and healthy mothers were (101.10, 108.50, P = 0.549) and (108.81, 107.29, P = 0.902), respectively.
Gestational diabetes in mothers with male and female fetuses was compared (Table 2).
| Variable | Gestational Diabetes | Total | P-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Gender | 0.800 | |||
| Female | 14 (48.3) | 94 (50.8) | 108 (50.5) | |
| Male | 15 (51.7) | 91 (49.2) | 106 (49.5) | |
| Total | 29 (100.0) | 185 (100.0) | 214 (100.0) | |
Fetal gender did not significantly affect the incidence of gestational diabetes in mothers (P = 0.800).
The difference between the fetal heart rate in the first trimester and after the glucose tolerance test in the fetuses of mothers with gestational diabetes was examined. In addition, the difference between the fetal heart rate in the first trimester and after the glucose tolerance test in the fetuses of healthy mothers was investigated (Table 3).
| Gestational Diabetes | N | Mean ± SD | P-Value |
|---|---|---|---|
| The difference between FHR in the first trimester and after the glucose tolerance test | |||
| Yes | 29 | 19.414 ± 10.95 | < 0.001 |
| No | 185 | 17.600 ± 10.131 | < 0.001 |
Abbreviation: FHR, fetal heart rate.
There was a significant difference between fetal heart rate in the first trimester and after the glucose tolerance test in mothers with gestational diabetes (P < 0.001). Moreover, there was a significant difference between the fetal heart rate in the first trimester and after the glucose tolerance test in healthy mothers (P < 0.001).
The results of logistic regression studies showed that fetal heart rate cannot predict gestational diabetes. Only maternal age (OR = 1.077, CI 0.95: 1.006 - 1.154) can predict gestational diabetes among all the investigated variables (mother’s age, fetal age, fetal heart rate, fetal CRL, and NT). The risk of developing gestational diabetes increases by 0.077 times every year of maternal age increase.
5. Discussion
The current study was conducted on 214 pregnant women to investigate the relationship between fetal heart rate in the first trimester and gestational diabetes. The fetal heart rate of the studied mothers was measured once in the first trimester and once after the glucose tolerance test. According to the glucose tolerance test, 29 pregnant mothers had gestational diabetes, while 185 were healthy. The results showed that the average age of mothers with gestational diabetes was significantly higher than healthy mothers. The average rank of fetal age in mothers with gestational diabetes was significantly lower than in healthy mothers. About 48.3% of the fetuses of mothers with gestational diabetes were female, and 51.7% were male. There was no significant difference in gestational diabetes in mothers with male and female fetuses. In addition, there was no significant difference between CRL and NT mean rank in fetuses of mothers with gestational diabetes and healthy mothers.
According to logistic regression studies, fetal heart rate does not predict gestational diabetes. Only the mother’s age could indicate gestational diabetes among all the investigated variables. Surveys showed that the risk of developing gestational diabetes increases by 0.077 times every year of maternal age increase. There was a significant difference in FHR in the first trimester and the time of glucose tolerance test in both group of pregnant women. This difference was slightly higher in mothers with gestational diabetes than healthy mothers.
Despite significant increases in FHR in both groups, the rise in FHR was not different in fetuses of healthy and diabetic mothers. Buscicchio et al. observed that baseline FHR was significantly higher in fetuses of diabetic mothers than their healthy counterparts in examining fetal heart rate in mothers with gestational diabetes (GDM) (13). Gameraddin et al. observed that maternal diabetes does not affect FHR in assessing FHR in the third trimester and comparing FHR in female and male fetuses. In addition, there was no significant difference between FHR in male and female fetuses (14). The results of Sirico et al. showed that the effect of gestational diabetes on FHR in weeks 11 - 14 of pregnancy is related to pre-gestational diabetes and CRL, but it is not related to mothers’ BMI, maternal age, gestational age, BPD and NT. The results showed that FHR in the first trimester in fetuses of diabetic and healthy mothers is more (12). Sirico et al. investigated the role of fetal heart rate in the first trimester of pregnancy (11 - 14 weeks) in predicting gestational diabetes. The results showed that the first trimester FHR strongly predicts gestational diabetes. According to the results, a threshold of 162 beats per minute predicted GDM with high detection accuracy and NPV (15).
The difference between the results and some previous studies could be due to the difference in the number of samples or the role of different genetics in diabetes and, as a result, the different prevalence of diabetes in different races (5).
One of the limitations of the present study was the non-cooperation of pregnant women in all stages of the study, and another limitation was the lack of time. Therefore, a large number of mothers were excluded from the study. More sampling was not possible due to the limited study time. Thus, another person should continue the study longer to reach an acceptable sample size and better results.
5.1. Conclusions
The results of logistic regression studies in predicting gestational diabetes showed that fetal heart rate cannot predict gestational diabetes. The only variable that could indicate gestational diabetes was the mother's age. Surveys showed that the risk of developing gestational diabetes increases by 0.077 times every year of maternal age increase.