1. Background
Infertility is a common clinical disorder in 13 - 15% of couples worldwide, which is defined as a conception failure following one year of unprotected sexual attempts (1, 2). The main infertility-related factors are spermatogenesis and oogenesis disorders, as well as genetic and environmental issues (3-6). In addition, it has been suggested that several factors directly contribute to infertility, including ovulation disorders, oviduct obstruction, peritoneal cavity complications (such as abdominal adhesions and endometriosis), pelvic tuberculosis, puerperal infections, and uterine cervix illnesses (7). Ovulation-stimulating drugs and ART are proposed as accepted remedies for promoting pregnancy rate and successful birth in humans (8).
ART is a multistep process, including intrauterine insemination (IUI) (9), transvaginal oocyte retrieval (OCR) (10), in vitro fertilization (IVF) (11, 12), intra-cytoplasmic sperm injection (ICSI) (11), gamete intra-fallopian transfer (GIFT) (12), zygote intra-fallopian transfer (ZIFT) (13), in vitro maturation (IVM) (14, 15), and assisted zona hatching (AZH) (16). In vitro fertilization is an assisted reproductive technique, which is widely used as the first line of infertility treatment and increases the pregnancy rate to 40 - 50% or even 60% (17). Various interfering factors appear to be involved in IVF failure, including low reception capacity of the endometrium, embryonic malformation, and immunological responses (17). According to studies, some factors have been reported as the negative outcomes of ART, like increased maternal metabolic complications (such as diabetes) and increased fetal disorders (such as metabolic and congenital malformations) (18). Fertility is a vital process that is triggered by the penetration of hereditary material of sperm into an oocyte to produce pronucleus and blastomeres. A 16-cell morula can enter the uterine cavity on the 4th day by the high aggregation of blastomeres on the 3rd day of gestation. Then, on the 5th day, the constituted blastocyst penetrates the endometrium in an implantation process (19). In vitro fertilization contains various laboratory stages, including hormone administration for hyper-stimulation of ovaries to oocyte production, collection of eggs (oocyte retrieval), sperm injection, fertilization, embryo culture, and finally, embryo injection (embryo transfer) (20). Embryos are commonly transferred in the cleavage stage (2 - 3 days after fertilization) with 2 - 8 cells or blastocyst stage (on days 5 - 6) (20-25).
According to the published articles, there are contradictions between embryo selection and transfer in proper stages, which are associated with various human diversities such as lifestyle, diet, invisible illnesses, inheritance, and mental beliefs.
2. Objectives
In the present study, the concept of the identical population (IP) is introduced to remove the influential and intervening variables and is traced to embryo transfer in stages of cleavage (2-cell) and blastocyst according to the IP concept.
3. Methods
3.1. Ethics Statement
The present study was approved by the Ethics Committee of the Kermanshah University of Medical Science, Iran (approval no: IR.KUMS.REC.13.01.2015).
3.2. Animals
The mice were selected based on the IP protocol. The animals were purchased from the animal house (affiliated with the Razi University, Kermanshah, Iran) and housed in cages under standard laboratory conditions, including 12L:12D of photoperiod and 20 - 25°C environment temperature. Food and water were also available for all animals.
3.3. IP Criteria
Identical population criteria for animal selection were used to eliminate the possible interfering factors. Identical population items included Balb/c mice strain, 30 g of weight, and six and eight weeks of age for females and males, respectively (26, 27). Only male and female mice with the same parents and generation were employed for IVF protocol. In addition, the recipient-IVF mice were from the same generation of sperm and oocyte donor animals. All male and female animals with the same parents were separated after puberty to synchronize the sexual cycles (at eight and ten weeks for females and males, respectively).
3.4. Experimental Design
Two experimental groups of 2-cell and blastocyst were used in this study. In the first stage, 350 oocytes were gathered, of which 287 were fertilized. After embryo formation, 106 and 181 embryos were considered for the 2-cell and blastocyst stages, respectively. Finally, 75 embryos in the 2-cell stage (35, 19, 21 embryos with grades A, B, and C, respectively) and 71 embryos in the blastocyst stage (24, 27, 20 embryos with grades A, B, and C, respectively) were transferred. Each experimental step was described in detail.
3.5. Sperm Preparation
The male mice with the age of 12 weeks were sacrificed by cervical dislocation on the day of the IVF procedure for sperm preparation. The tail of the epididymis was cut into the sperm dish after abdominal dissection. A slight incision was made on the epididymis, and ambulatory sperms were aggregated at the apex of each drop for sperm release. The sperm dish was incubated at 35°C with CO2 5% for at least 15 min. The sperms were observed through an inverted microscope to calculate the motility. The sperm smear was stained by Papanicolaou staining to determine the normal morphology. The viability of sperm was studied by trypan blue staining. Sperms with 80% viability, 70% motility, and 70% normal morphology were included to perform IVF (28).
3.6. Collection of Metaphase II Oocytes
Female mice aged eight weeks were super-ovulated by i.p. injection (10 IU) of pregnant mare serum gonadotropin (PMSG) and received 10 IU of human chronic gonadotropin (HCG) intraperitoneally. About 14 hours later, the injected mice were killed by cervical dislocation for oocyte collection. Following the laparotomy procedure, the oviducts were cut and placed into a 60mm dish containing T6 medium. Finally, 350 metaphase II (MII) oocytes were selected for IVF (28).
3.7. Sperm Transfer Into the IVF Dish
In this step, the ambulatory sperms at the top of the drops were transferred into the IVF drop using a mouth pipette. Then, MII oocytes were transferred into the IVF drop and were incubated at 37°C and CO2 5%. Approximately, there are 4 - 5 oocytes in each 50 μL drop. The mice were eluted in decline and transferred into the IVF drop to eliminate the additional sperms surrounding the oocytes 6 - 8 h after IVF and pronucleus formation.
3.8. Evaluation of Embryos in Blastocyst and Cleavage Stages
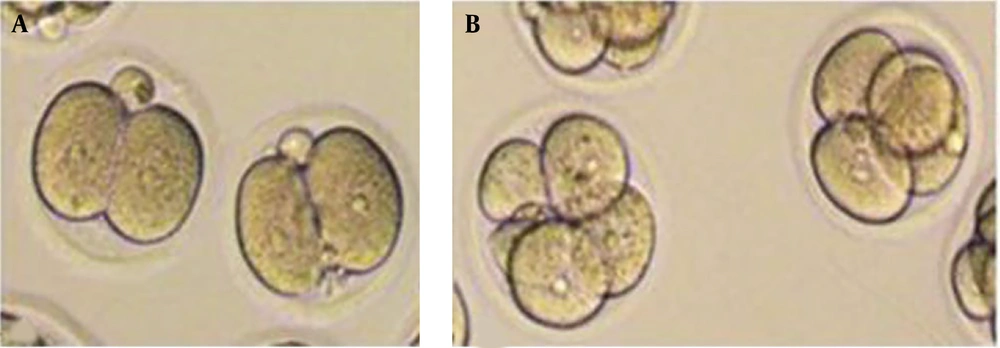
The IVF dishes were investigated by an inverted microscope 24 hours after the IVF procedure to examine the formation of 2-cell embryos in the cleavage stage (Figure 1). According to scoring criteria, 2-cell embryos were classified into A, B, and C groups (29). The embryos were graded based on the fragmentation level and blastomeric size in the cleavage stage. Grade A: Equal-sized blastomeres with no fragmentation; grade B: Blastomeres with a slight difference in size and 10% fragmentation; grade C: Blastomeres with a more considerable difference in size and 20% fragmentation. The IVF dishes were examined daily (twice a day at 6 AM and 6 PM) to examine the development of embryos into the blastocyst stage, and developed embryos were transferred into new drops. The embryos were transferred to a recipient mouse after 96h and the formation of blastocysts. The embryo grading at the blastocyst stage was performed based on blastocyst development, cellular mass evolution, and trophectoderm organization (30). Grade A: Expanded blastocysts with distinctive cell mass and thin organized trophectoderm; grade B: Less expanded blastocysts with distinct cell mass and few wide trophectoderm; grade C: Less extensive blastocyst, indistinctive cell mass, with irregular flat cells of trophectoderm.
3.9. Embryo Transfer
A connector connected a blue peripheral venous catheter (+26) with 0.9 mm internal diameter and 25 mm length to a vein scalp catheter to transfer an embryo. In addition, an insulin syringe (piston-free) was connected to another end of the catheter. One hollow rod with 4 mm internal diameter and 10 mm length was used instead of a speculum. T6 medium (8 mg/mL BSA) with two folds of serum was prepared as a fluid environment for embryo transfer. Then, one drop (10 μL) was dripped on a 60 mm dish. The embryos were transferred from the IVF dish into the drop using a mouse pipette under a stereomicroscope. Following a speculum insertion into the vaginal canal, the catheter was installed, and embryos were slowly transferred.
3.10. Assessment of the Live Birth Rate
The index of live birth rate was explained as the number of embryos implanted into the endometrium precisely five days after embryo transfer. The implantation sites were revealed visually by intravenous injection of 0.4% Trypan Blue solution on the 5th day after embryo transfer. The recipient mice were sacrificed by cervical spine displacement 15 minutes after injection. Uterine horns were cut and studied by a stereomicroscope. Implantation sites were stained due to increased vessel penetration.
3.11. Statistical Analysis
The data were analyzed using GraphPad Prism (version 9.0.0) software. Fisher’s exact test was used to compare the groups. The statistical significance level was determined as P < 0.05, and the data were presented as the mean ± SEM.
4. Results
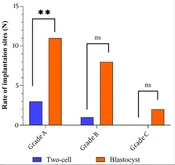
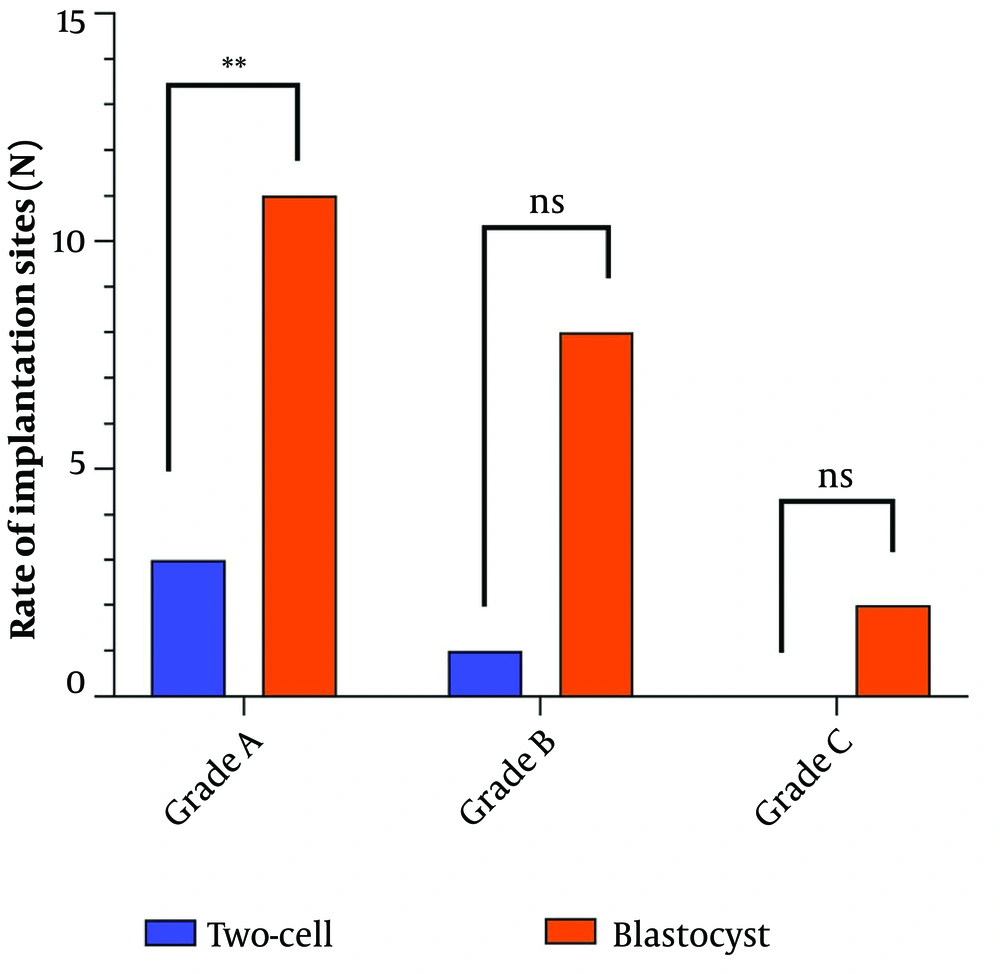
The total number of transferred embryos from each grade and the number of implantation sites are summarized in Table 1. The results indicated that the rate of implanted embryos in the blastocyst stage increased compared to the 2-cell stage.
| Embryos and Grade | Rate of Transferred Embryos | Rate of Implantation Sites | Rate of Live Births |
|---|---|---|---|
| Two-cell | |||
| A | 35 | 3 (8.57) | 1 (2.85) |
| B | 19 | 1 (5.26) | 0 (0) |
| C | 21 | 0 (0) | 0 (0) |
| Blastocyst | |||
| A | 24 | 11 (45.83) | 5 (20.83) |
| B | 27 | 8 (29.62) | 3 (11.11) |
| C | 20 | 2 (10) | 1 (5) |
Rate of Transferred Embryos, Implantation Sites, and Live Births in Grades A, B, and C in Two-Cell and Blastocyst Stages a
4.1. Determination of Successful Implantation Rate Following Grade A Embryo Transfer
In grade A, 35 embryos in the 2-cell stage and 24 embryos in the blastocyst stage were transferred to the recipient mice. The results showed that the rate of implanted embryos in the blastocyst stage increased significantly compared to the 2-cell stage. Three implantation sites for 2-cell transfer (8.57%) and 11 implantation sites for blastocyst transfer (45.83%) were observed with a significant difference (P = 0.001) (Table 2 and Figure 2).
| Variables | Group 1 | Group 2 | P-Value |
|---|---|---|---|
| Implantation sites | Two-cell grade A | Blastocyst grade A | 0.001 |
| Two-cell grade B | Blastocyst grade B | 0.064 | |
| Two-cell grade C | Blastocyst grade C | 0.231 | |
| Live birth | Two-cell grade A | Blastocyst grade A | 0.036 |
| Two-cell grade B | Blastocyst grade B | 0.256 | |
| Two-cell grade C | Blastocyst grade C | 0.487 |
Significance Levels of the Same Grades in Both Groups of 1 and 2 in the Blastocyst and Two-Cell Stages
4.2. Determination of Successful Implantation Rate Following Grade B Embryo Transfer
According to the results, 19 embryos in the 2-cell stage and 27 embryos in the blastocyst stage of grade B were transferred to the recipient mice, in which the rate of implanted embryos in blastocyst increased compared to the 2-cell stage. One implantation site for 2-cell transfer (5.56%) and eight for blastocyst transfer (29.62%) were observed in this grade. However, this difference was insignificant (P = 0.064) (Table 2 and Figure 2).
4.3. Determination of Successful Implantation Rate Following Grade C Embryo Transfer
Based on the results of grade C, 21 embryos in the 2-cell stage and 20 embryos in the blastocyst stage were transferred to the recipient mice. The rate of implanted embryos in the blastocyst stage was increased compared to the 2-cell stage, and no implantation sites were observed for 2-cell transfer. However, two implantation sites for blastocyst transfer (10%) were found. The difference was insignificant (P = 0.231) (Table 2 and Figure 2).
4.4. Rate of Live Birth in Implanted Embryos
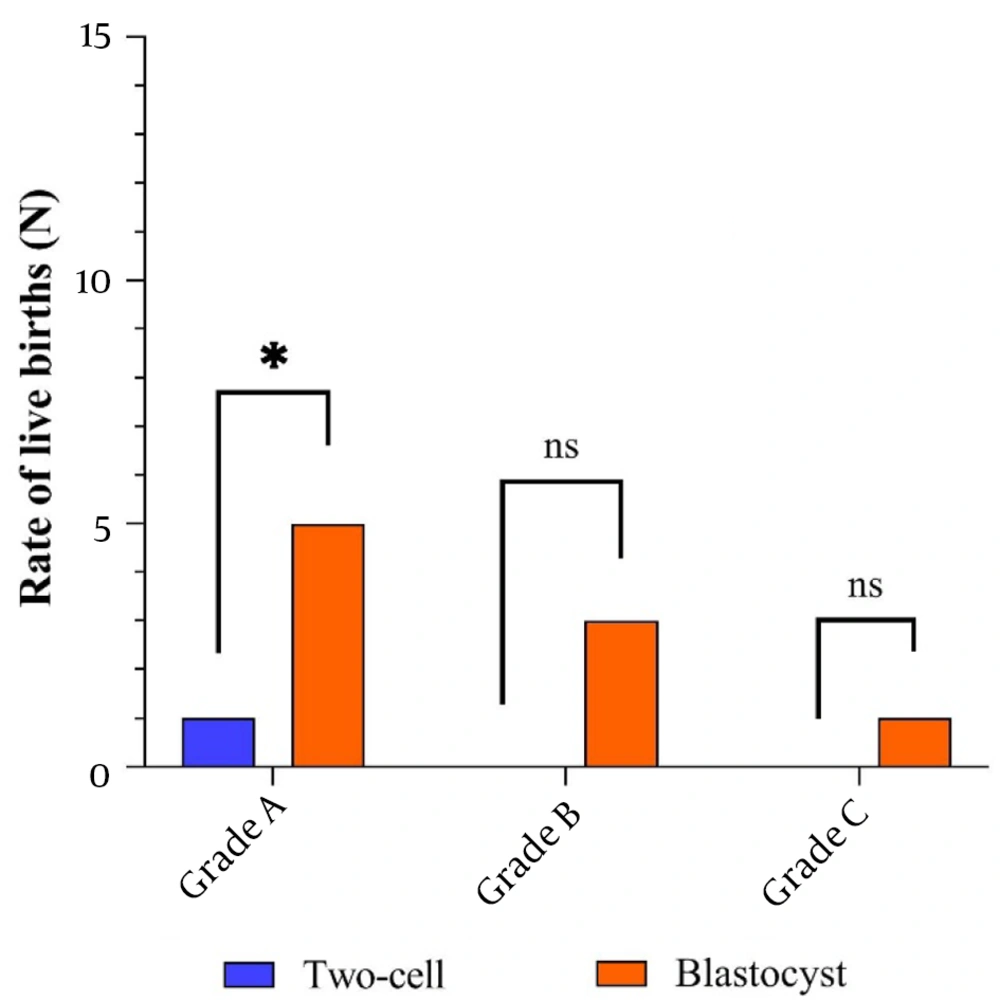
A total number of live births in all grades was assessed. The results showed that the live birth rate in the blastocyst stage significantly increased compared to the 2-cell stage. As shown in Table 1, in the blastocyst stage, 5 (20.83%), 3 (11.11%), and 1 (5%) live birth for grades A, B, and C, respectively, and only (2.85%) 1 live birth for grade A in the 2-cell stage were detected. However, no live birth was observed in grades B and C in the 2-cell stage (P = 0.036, P = 0.256, and P = 0.487 for grades A, B, and C, respectively) (Table 2 and Figure 3).
5. Discussion
According to studies, there are numerous contradictions in the proper timing of embryo transfer in IVF procedures. Besides, the intrauterine embryo transfer approach on the 2nd, 3rd, and 5th days after transvaginal oocyte retrieval (TVOR) is widely accepted today (20-25). Embryo transfer is a final and indispensable step in the IVF procedure. The embryo transfer stage is an essential factor affecting the implantation rate and the success level of IVF (20). The present study aimed to investigate the rate of successful implantation and live birth following the transfer of mice embryos in cleavage and blastocyst stages. Moreover, the IP concept of the present study was explained to eliminate the probable influential genetic and environmental variables. Unlike human studies, animal models can prepare an identical population, including primary genetic sources, nutritional habits, and environmental factors (31). The obtained results suggested that the rates of implantation and live birth in all blastocyst transfer grades are higher than in 2-cell embryo transfer. Generally, the last embryonic stage before implantation is the blastocyst phase, which seems appropriate for embryo transfer. The rationale for extending embryo culture to the blastocyst stage is the improvement of uterine and embryonic synchronicity and the provision of the chance of self-selection by viable embryos. Thus, these processes can lead to proper live birth rates in embryo transfer. Various studies have developed in embryo transfer in cleavage and blastocyst stages that have reported contradictory results (20-25, 32, 33). For example, Karaki et al. reported that embryo culturing of the blastocyst stage can result in a considerably higher implantation rate than embryos transferred on day 3 (32). Cheng et al. cultured embryos (extracted from ICR mice) in an HTF medium to form blastocysts and classified them into three groups based on the quality rating. The data indicated that the high quality of blastocysts is associated with an increased implantation rate, which aligns with our results using Balb/c mice and T6 medium (34). Kang et al. compared the effects of blastocyst and morula transfer and reported that both stages could lead to a similar implantation rate, contrasting our findings (35). In addition, a systematic review and meta-analysis study published in 2017 found no priority in the cleavage stage of embryo transfer compared to the blastocyst stage in clinical trials (36). Another study demonstrated a significant increase in live birth rates and clinical pregnancy using blastocyst compared to the cleavage stage in patients undergoing frozen-thawed embryo transfers (FET) (25). On the other hand, it was concluded that the blastocyst transfer could reduce the number of efficient embryos due to safety concerns such as perinatal mortality and early preterm birth (20, 37-39). Recently, a published study reported that the cumulative pregnancy rate is similar among pregnant women with 1 and 2 embryos on day 3, regardless of whether the embryo is transferred at the cleavage or blastocyst stages (40). However, Li et al. evaluated the morula transfer on day four and the blastocyst transfer on day 5 and reported that embryo transfer in the morula stage is a more comfortable, flexible, and applicable method for embryo transfer (24). The implantation rate from embryo transfer in the stages of cleavage and blastocyst was also evaluated by Mangalraj et al. Their results showed that the level of blastocyst implantation (40.16%) was different from the cleavage stage (11.43%) (41). Thus, there are contradictions in the appropriate stages of embryo transfer in the literature, requiring more detailed research in this field. In the present study, the small size of animals limited the availability and manipulation of cervical embryo transfer. Otherwise, only pure grades (A, B, and C) were included to eliminate interfering factors. Besides, the interstitial grades consisting of (A, B) and (B, C) were not calculated, although possible live births existed in interstitial grades. In addition, the embryos with rapid and delayed development were excluded in this study because they could affect the live birth rate. Thus, it is recommended that a large sample size be used for future studies to detect the changes with more precision.
5.1. Conclusions
Based on the results, the implantation rate following embryo transfer in the blastocyst stage could significantly increase the rate of live birth compared to the cleavage stage. Thus, the results of the present study can be considered successful embryo transfer to improve implantation and pregnancy rates in IVF.