1. Background
Cancer is one of the most risky sicknesses and a primary reason for the loss of life worldwide (1). Oral squamous cell carcinoma is the most common head and neck SCC, accounting for 90% of oral cancers (2). Conventional treatment of OSCC includes surgery, radiotherapy, and chemotherapy. Oral cancer treatment is generally surgical, followed by radiotherapy or chemotherapy (3). However, the 5-year survival rate of OSCC remains in 40 - 60% of cases despite rapid advances in treatment technologies and extensive research (4, 5). In addition, significant functional complications are created in the surrounding natural tissues due to the aggressiveness of these treatments by reducing the patient’s quality of life (6). Extensive studies have been conducted on developing effective and low-risk targeted treatments, such as treatments based on immunological methods, cell therapy, gene therapy, and nanoparticle-based methods (7). New methods of diagnosis and treatment have been developed to help cancer patients continue their lives. Today, nanoscience has contributed significantly to accurate and early diagnosis and delivery of anticancer drugs to cancer cells (8-10). Novel nanomaterials are a promising strategy to overcome therapeutic resistance, such as malignancy and multidrug resistance. Cancer is a challenge for global health due to the increase in the disease rate and related economic burden (11). Appropriately engineered nanoparticles can isolate pharmaceuticals from the circulatory system and inhibit renal elimination. The mentioned nanoparticles have enhanced the assimilation of anticancer agents at the intended sites and curtailed indiscriminate harm to native tissues triggered by unbound drugs and augmenting the permeability and retention effect (EPR) (12). Studies have shown that the anticancer activities of metal oxide nanomaterials among different nanomaterials are auspicious (13, 14). Magnesium oxide nanoparticles have garnered more attention in contemporary times than other metal oxide nanoparticles widely used in various domains. The primary reasons are the materials’ low density, commendable performance, recyclability, and non-hazardous nature. Consequently, the nanoparticles exhibit a propitious configuration for biological implementations (15-17). Magnesium oxide nanoparticles possess potential as a robust and efficacious anticancer agent (18). Nonetheless, a significant constraint of employing metal nanoparticles is their pronounced inclination to aggregate. Therefore, stabilizing agents, such as polymers, can inhibit undesirable aggregation of nanoparticles (19). Polyvinyl alcohol (PVA) is an appealing hydrophilic artificial polymer with numerous lively hydroxyl practical groups, which is broadly used in the prescription of drugs and biomedicine with essential functions in biocompatibility, biodegradability, low cytotoxicity, and bactericidal effects (20). The effect of PVA/MgO nanocomposite on oral cancer cells has not been evaluated before, as stated in the abovementioned explanations.
2. Objectives
Considering the importance of mitigating adverse effects and expediting the treatment process, the present research investigated the anticancer potential of polyvinyl alcohol/MgO nanocomposite in human oral cancer cells.
3. Methods
The present study was conducted upon the approval and adoption of the ethics code (IR.KUMS.REC.1399.798) at the advanced dental science research center of Kermanshah University of Medical Sciences.
3.1. Materials
The utilized materials, namely polyvinyl alcohol (PVA), magnesium nitrate (Mg (NO3)2), and sodium hydroxide (NaOH), were procured from Sigma. Furthermore, the essential materials for cell culture studies, such as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT), Fluorescent probe 2,7-dichlorofluorescein diacetate (DCF-DA), triton X-100, rhodamine 123, and Caspase-3/7 Detection Kit were purchased from Sigma (St Louis, MO, USA). The acquisition of fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM-F12) was accomplished through procurement from Gibco (Gibco, Grand Island, NY, USA).
3.2. Synthesis of Magnesium-Oxide Nanoparticles
The present study used the co-precipitation method to synthesize magnesium oxide nanoparticles. For this purpose, 0.2 mol of Mg (NO3)2 and 0.2 mol of NaOH were prepared and stirred on a stirrer for 90 min. Separated components were centrifuged, washed, dried in an oven, and then centrifuged again to remove impurities. The process was concluded by subjecting the magnesium hydroxide powder to calcination in an oven at a temperature of 450°C, producing a white powder of magnesium oxide nanoparticles (21).
3.3. Preparation of Polyvinyl Alcohol-Magnesium Oxide Nanocomposite
In this study, PVA biopolymer was prepared commercially. About 3 mg/mL PVA and 4 mg/mL MgO NPs were distilled into 100ml of water and stirred with a magnetic stirrer for 60 minutes at room temperature to synthesize PVA/MgO nanocomposite. Then, the two compounds were dispersed via an ultrasonic homogenizer for 15min at 42°C. MgO nanoparticles are added dropwise to the polymer mixture and stirred for 60 min. This solution was then dispersed via ultrasonic homogenizer at 42°C for 15 minutes. Finally, the organized nanocomposite was separated via centrifugation, washed with distilled water for three instances to cast off unreacted and residual species, and located in an oven at 60°C for 24 h to dry. The acquired dry sediment separates from the box with a spatula and floor-to-powder (22).
3.4. Characterization
The present study used SEM examination to analyze the dimensions and configuration of the synthesized nanocomposite and nanoparticles. The conformation and configuration of the synthesized nanocomposite were scrutinized using a TESCAN MIRA3 SEM, which operated at a voltage of 30 kV with a magnification of 2 μ.
3.5. Cell Culture
The present study used oral papilloma cancer cells to perform cellular studies. Thus, the KB cell line was purchased from the Pasteur Institute of Iran-Tehran. The cells were placed in suitable conditions for growth, including temperature of 37°C, humidity of 95%, and atmosphere containing 5% CO2. DMEM-F12 cell culture medium containing 10% (v/v) FBS in inactivated form and 1% penicillin and streptomycin were used to culture the cells until reaching the appropriate density (70 - 80%).
3.6. Cytotoxic Study
The MTT method was used on the KB cell line to check the concentration of nanocomposite with the highest anticancer properties. Hence, 20,000 cells were cultured in each well of a 96-well plate to a final volume of 200l and placed in an incubator. Polyvinyl alcohol -magnesium oxide nanocomposite was applied to the cells 24 hours after seeding. The environment of the wells was empty after the intended treatment and inverting the plate gently on the filter paper, and the cells were washed with 200 μL of PBS. Then, MTT yellow solution in a volume of 150 μL and complete culture medium was mixed at a ratio of 1: 10 and added to each well, and the plate was placed in an incubator away from light for 4h. Then, 100 μL of DMSO answer was brought to every properly, and the favored plate was shaken for 10 min until the crystals were dissolved. Finally, the quantity of mild absorption changed into measured at a wavelength of 570 nm. The common absorption of three wells similar to every remedy changed compared to the manipulated group. In the subsequent step, IC50 attention changed into calculated via way of means of Prism software.
3.7. Intracellular ROS Measurement
Polyvinyl alcohol-magnesium oxide nanocomposite was used to investigate ROS changes in KB cells after exposure to its IC50 concentration for 24 h. DCF reagent was added to the wells after 24 hours (group treated with nanocomposite and control group). About 800 μL of Triton-x100 was added to each well after incubating the plates for 45min and then washing with PBS, and the plates were kept at 4°C for 30 min. The contents of the plates were centrifuged for 15 minutes after being transferred to a microtube. Finally, the fluorescence of the samples was measured at an excitation wavelength of 488 and an emission wavelength of 510 nm using a microplate lidar (Bio-Tek, ELX 800, Winooski, VT) (7).
3.8. Mitochondrial Membrane Potential Measurement
The present study used rhodamine 123 fluorescence dye to investigate the changes in mitochondrial membrane potential (∆Ψm) (MMP) in KB cells after exposure to IC50 concentration of PVA-magnesium oxide nanocomposite. The cells were treated with IC50 concentration of nanocomposite for 24 h after culture in a six-well plate. Next, 20 μL of Rhodamine 123 was added to the cell culture medium and incubated for 45 min. PBS was added to each of the wells for washing after the incubation time, and 1mm of Triton-x100 was added to the cells, and they were incubated for 30 min at 4°C. Next, the cells were centrifuged at 13000 rpm. Finally, the fluorescence value of the samples was measured at the excitation wavelength of 488nm and the emission wavelength of 520nm using a microplate lidar device (23).
3.9. Caspases Assay
Caspase 3 and 7 activity assay kit was used to investigate the apoptosis process in KB cells when exposed to PVA-magnesium oxide nanocomposite at IC50 concentration. Briefly, the cells were cultured in 12-well plates and treated with IC50 concentration of nanocomposite for 24 h. The cells were trypsinized and centrifuged at 12000 RPM for 7 min after 24 h of incubation. Then, 100 μL buffer was added to the samples and incubated for 20 min at 4°C. Next, the samples were washed and centrifuged for 15 min at 12,000 rpm. Then, 50 μL of the supernatant solution was taken from each sample and transferred to the well of 96 Well Plate with 55.5 μL of the working solution. The samples were read at 405 nm using an ELISA reader at the end of absorption (23). Working solution: Caspase buffer: 50 ul, DTT: 0.5 ul, caspase substrate: 5ul.
4. Results
4.1. SEM
SEM analysis was used to assess the structure and morphology of PVA-MgO nanocomposite (Figure 1). Spherical structures with regular dispersion of MgO nanoparticles are shown in the figure. In addition, the proper dispersion of nanostructures in the PVA matrix confirms the appropriate synthesis of PVA-MgO nanocomposite.
4.2. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Assay
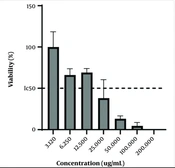
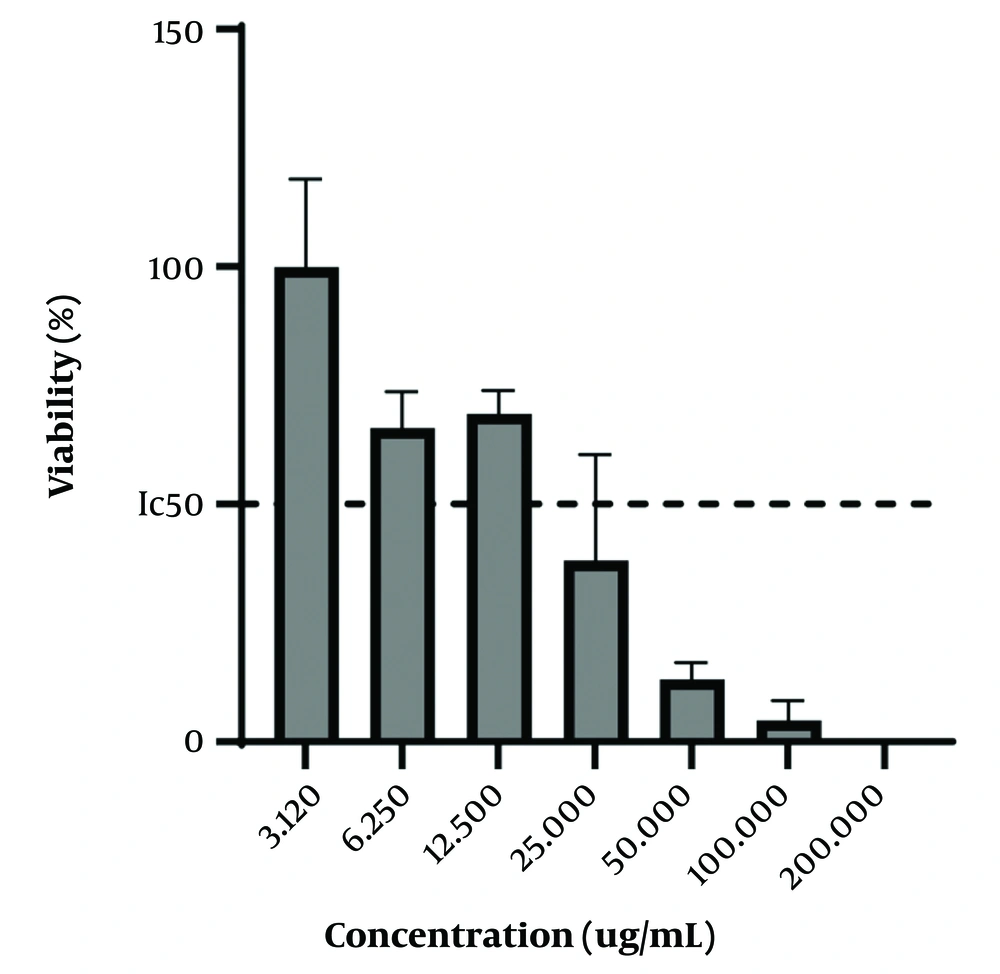
In the present study, the MTT method was used on the KB cell line to investigate the concentration of PVA-MgO nanocomposite with the highest anticancer properties. The obtained results showed that the nanocomposite synthesized in the concentration, range between 12.5 - 3.12 ug/mL, had minor anticancer activity so that at the concentration of 3.12 ug/mL, and almost 100% of the Cancer cells of the KB category were alive. In addition, the synthesized nanocomposite had the maximum anticancer activity in the concentration range of 25 - 200 ug/mL, in such a way that the survival rate of KB-type cancer cells is less than 50% in the mentioned concentrations. The obtained results are shown in Figure 2.
4.3. ROS Measurement
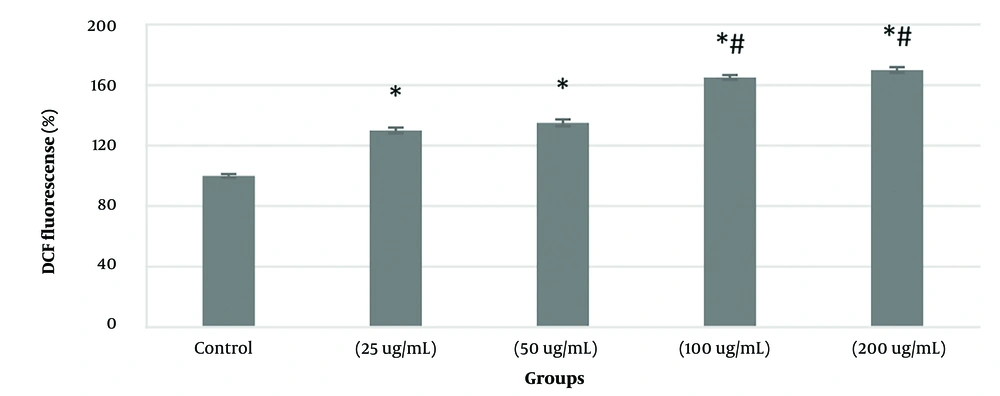
KB cancer cells with four concentrations of 25, 50, 100, and 200 ug/mL were treated within 24 h to investigate the changes in the level of ROS in KB cancer cells treated with PVA-MgO nanocomposite based on the results of the MTT (IC50). The results showed that the treatment of KB cancer cells with the synthesized nanocomposite in the studied concentrations significantly (P < 0.05) compared to the control group (100%) increased the amount of ROS in these cells. The results also showed that the changes in the amount of ROS in these cells have improved somewhat in a dose-dependent manner, so that the amount of ROS in the concentration of 100 and 200 μg/mL was significant (P < 0.05) compared with the concentration of 25 µg/mL, it has increased (Figure 3).
4.4. Mitochondrial Membrane Potential Measurement
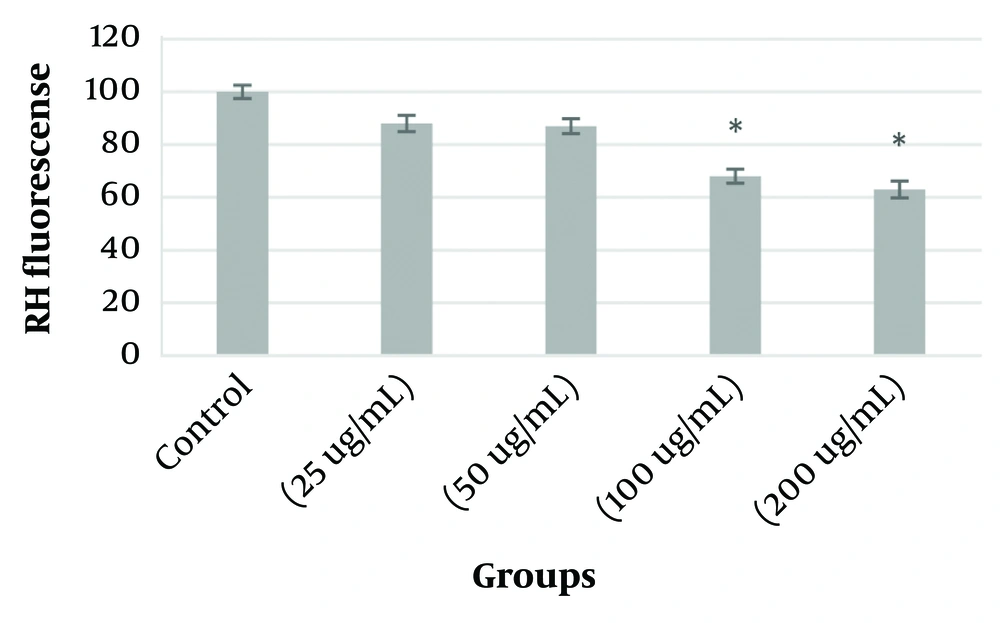
KB-type cancer cells were treated with four different concentrations of PVA-MgO nanocomposite based on the obtained IC50 to investigate changes in mitochondrial membrane potential (MMP). The results showed that mitochondrial potential decreased in these cells after exposure of KB cancer cells to PVA-MgO nanocomposite. Hence, the decrease observed in 100 and 200 ug/mL concentrations was significant compared to the control group (P < 0.05). The lowest decrease was observed in the concentration of 200 µg/mL (Figure 4).
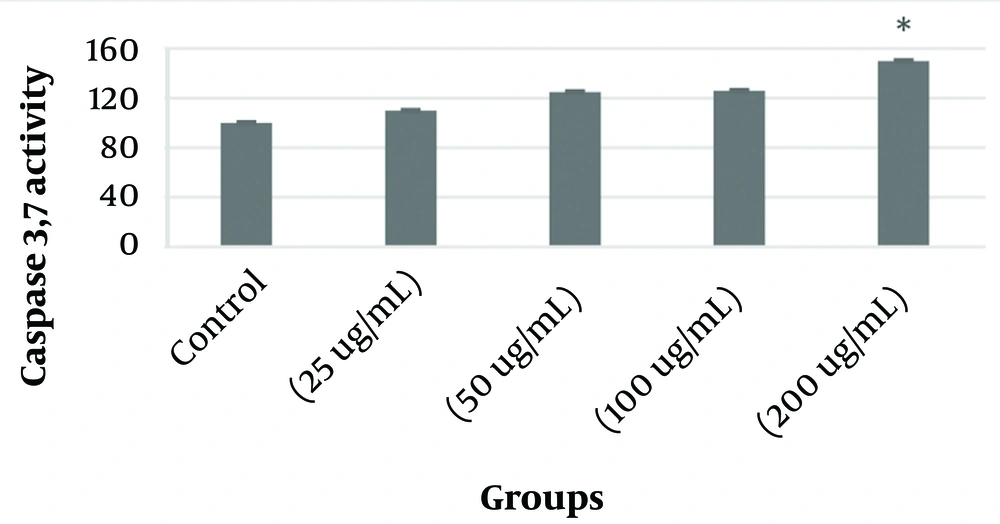
4.5. Caspases Assay
Figure 5 shows changes in caspases 3 and 7 activity in KB cancer cells after treatment with different concentrations of PVA-MgO nanocomposite. The results indicated that treating KB cells with the synthesized nanocomposite increased the activity of caspases 3 and 7 in these cells. The significant activity level in this study was at 200μg/ml, which, compared to the control group (P < 0.05).
5. Discussion
Oral cancer is one of the most unusual and deadly sicknesses and has long been a severe difficulty for public fitness worldwide (24). Squamous mobileular carcinoma of the oral hollow space is the maximum not unusual place shape of most oral cancers, accounting for greater than 85% of cases. Although early detection of most oral cancers is vital, maximum sufferers are recognized for the ultimate degree of the disease, which ends up in a terrible prognosis (25). Although oral cancer is commonly associated with alcohol and tobacco use, one study found poor oral hygiene to be an important risk factor for non-smokers and non-alcoholics (26). Periodontitis has been a facilitating characteristic of most oral cancer development in oral squamous mobileular carcinoma (OSCC) as it’s far a not unusual place inflammatory disorder affecting the oral cavity (27). Chronic periodontal infection is a longtime hazard element for most oral cancers because it has been proven that continual infection sufferers are at better risk of growing malignancy than healthful individuals (28). Oral squamous cell carcinoma (OSCC) limits treatment options with conventional approaches such as surgery, radiation, and chemotherapy, significantly affecting the patient’s quality of life. As a result, it is imperative to explore novel drug delivery strategies to facilitate the precise administration of medications, thereby minimizing adverse reactions and toxicity in individuals (29). Drug delivery structures primarily based on nanotechnology are satisfactory to have an answer and are essential for the unique concentration of most cancer cells. Nanoparticles, liposomes, exosomes, and cyclodextrins are nano-primarily based vendors for drug delivery (30). In the present study, the anticancer effects of PVA-Mgo nanocomposite were investigated against oral cancer cells (OSCC) in vitro. The outcomes acquired from the MTT assay confirmed that the synthesized nanocomposite inside the attention variety of 25-two hundred ug/mL has excessive deadly homes in opposition to most oral cancer cells. Similar studies have shown the anticancer activity of MgO nanoparticles in combination with other metallic and non-metallic nanoparticles in nanocomposites. A similar study showed that the synthesis of Ag-MgO nanocomposite is cost-effective and efficient and has potential applications in various fields, including anticancer activity. A synthesized nanocomposite was effective for anticancer activity and demonstration on H69PR lung cancer cells. It was shown that the nanocomposite made by reducing H69PR cells (IC50 = 190 μg/mL) has shown anti-cancer activity (31). A separate investigation demonstrated that MgO nanoparticles and GO/MgO nanocomposite display substantial cytotoxicity towards prostate cancer cell lines. The IC50 value of MgO nanoparticles and GO/MgO nanocomposite against prostate cancer cell lines (PC3) were determined as 86.7 and 11.17 μg/mL, respectively (32). In addition, some others have confirmed that MgONPs have substantial cytotoxicity in opposition to A549 lung most cancers mobileular traces in a dose-based way with an IC50 price of 37.5 ± 0.34 μg/mL (33). In addition, other studies have indicated the anticancer role of PVA in the form of nanocomposite and combination with other materials. A study investigated the cytotoxicity of PVA-cellulose-curcumin nanocomposite in laboratory conditions as a selective inhibitor of breast and liver cancer cell proliferation. The results showed that the viability of cancer cells decreased to 35 and 7% in MCF-7 and Huh-7 cells, respectively, at a high concentration (8 mg/mL) of PVA/CNCs/Curcumin nanocomposite (34). Reactive oxygen species (ROS) are especially reactive and are stable with diverse antioxidant defenses. Excessive mobile tiers of ROS cause harm to proteins, nucleic acids, lipids, membranes, and organelles, which can activate cellular loss of life techniques along with apoptosis. ROS performs an important position in cellular signaling and regulates the principle apoptosis pathways mediated by using mitochondria, loss of life receptors, and endoplasmic reticulum (ER) (35). the role of PVA-MgO nanocomposite in the apoptosis of oral cancer cells (OSCC), Intracellular ROS, Mitochondrial Membrane Potential, and Caspases 3,7 activity was investigated in this study considering the role of ROS and mitochondria in the apoptosis signaling pathway. The acquired effects confirmed that PVA-MgO nanocomposite uncovered to oral most cancer cells at specific concentrations of IC50 through growing ROS, lowering the mitochondrial membrane potential, and growing the hobby of caspases three and seven in those cells has precipitated mobile apoptosis. Studies have proven that the poisonous results of MgO NPs are especially because of the influential disturbances in oxidative pressure that cause apoptosis due to the buildup and internalization of nanoparticles and their interplay with mobile proteins consisting of Sod1 and p53, affecting structural integrity and function (36). In addition, other studies have confirmed that magnesium in the form of nanocomposite and in combination with platinum (Pt/MgO) has cytotoxicity on human lung and colon cancer cells (A549 and HT29, respectively). The results demonstrated that the Pt/MgO nanocomposite could decrease the expression of Bcl-2 and regulate tumor suppressor proteins like Bax and p53 in cancer cells. Additionally, the Pt/MgO nanocomposite could stimulate the production of reactive oxygen species, lower cellular glutathione levels, and raise lipid peroxidation. These findings suggested that the anticancer properties of the Pt/MgO nanocomposite are due to the triggering of oxidative stress and apoptosis (37). Additionally, studies have demonstrated that Pd/MgO nanocomposite exhibits more anti-proliferative effects in cancer cells than normal cells. In cancerous cells, Pd/MgO nanocomposite instigated apoptosis by enhancing caspase activities and prompting the release of cytochrome C. The anticancer properties of Pd/MgO nanocomposite were further augmented through the upregulation of Bax and p53 proteins and the concomitant reduction of Bcl-2 protein expression (38). In addition, chitosan/PVA nanofibers containing chitosan nanoparticles containing vanadium induced apoptosis and cell cycle inhibition in human skin cancer cells (39).
5.1. Conclusions
Based on the results, PVA/MgO nanocomposite has suitable anticancer properties against KB cancer cells. In addition, the results of the SEM analysis confirmed the proper synthesis of the studied nanocomposite. In general, PVA/MgO nanocomposite can be considered a promising candidate for anticancer applications in the future due to the appropriate binding ability of MgO nanoparticles and the multiple properties of PVA in improving the biological and physicochemical properties of the container structures.