1. Background
Vitamin D reduces the risk of microbial infections and mortality through several mechanisms, such as producing protective peptides and increasing the natural immunity of cells and adaptive immunity (1). Vitamin D deficiency prevents these mechanisms from working, making people susceptible to various infections, such as respiratory tract viruses (2). The effect of vitamin D supplementation on acute respiratory tract infection has been demonstrated in clinical trial studies. Studies have shown that regardless of gender, age, and the duration of the study, vitamin D supplementation reduced the risk of acute respiratory tract infection in all patients (3, 4).
The severe acute respiratory syndrome virus (SARS-CoV-2) and the causative agent of COVID-19 caused inflammation and irritation in the upper and lower respiratory system, resulting in severe acute respiratory syndrome. The symptoms of COVID-19 vary from mild or moderate with fever, dry cough, and fatigue to severe and even acute levels with shortness of breath, hospitalization in intensive care units (ICU), acute respiratory syndrome, multiple organ failure, and death (5). A significant inverse relationship was observed in 20 European countries between the serum concentration of 25-hydroxyvitamin D and the cases of COVID-19, the length of hospitalization, and the mortality rate. Vitamin D supplementation had beneficial effects in reducing the incidence and severity of COVID-19 by increasing the serum concentration of 25-hydroxyvitamin D (6).
Several studies have investigated the relationship between vitamin D deficiency and the mortality rate and length of hospitalization of COVID-19 patients (7). However, most of these studies have remained inconclusive or highlighted the need for further randomized controlled clinical trials. Similarly, there is limited evidence indicating the effect of vitamin D supplementation on improving clinical outcomes of COVID-19, unlike other respiratory diseases. Some studies have not indicated vitamin D supplementation's effectiveness in improving the clinical outcomes of COVID-19 patients (8-10). However, another study demonstrated the effect of a twofold reduction in the mortality of patients taking vitamin D supplements daily compared to non-users (11).
2. Objectives
Considering the heterogeneity in the available clinical results, the present study examined the effect of high-dose vitamin D on the hospitalization duration and the mortality rate of patients infected with COVID-19 hospitalized in the ICU.
3. Methods
The present study was a randomized, double-blind, placebo-controlled, and single-center clinical trial designed based on the Consolidated Standards of Reporting Trials statement under the Declaration of Helsinki. This study was conducted on COVID-19 patients admitted to Golestan Hospital, Kermanshah University of Medical Sciences, Iran, after obtaining approval from the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran (IR.KUMS.MED. REC.1400.069) and being registered in IRCT.IR (Iranian Registry of Clinical Trial) (IRCT20170827035936N2). An informed consent form, including the trial protocol and the implementation method of the plan, was obtained from the patients before they participated in the study.
3.1. Participants
The study subjects consisted of patients with COVID-19 hospitalized in the ICUs of Golestan Hospital, who were included in the study from November 2021 to April 2022.
3.2. Inclusion Criteria
Patients over 18 years with confirmed SARS-CoV-2 infection by polymerase chain reaction or involvement seen in computed tomography scan, admitted to the ICU, with 25-hydroxyvitamin D levels of less than 30 ng/dL and without underlying diseases or with at least one underlying disease, such as diabetes, blood pressure, and heart failure, were included in the study.
3.3. Exclusion Criteria
Patients who were unable to sign the consent form, pregnant and lactating, with a history of less than 24h in the ICU, taking vitamin D supplements (>1,000 IU/D) in the past month, hypercalcemia (calcium ≥ 10 mg/dL), cancer and under treatment with radiotherapy, chemotherapy, immunotherapy, with hypoparathyroidism, hypercalciuria, sensitivity to vitamin D, kidney failure (creatinine ≥ 2 mg/dL) and under dialysis, nephrolithiasis, and liver failure and cirrhosis, receiving mechanical ventilation at admission, participated in other studies, and were unwilling to continue cooperation were excluded from the study.
3.4. Randomization and Study Interventions
Patients were placed in the treatment group (300,000 IU vitamin D3 + standard treatment) or the control group (placebo + standard treatment) with a parallel design and a ratio of 1: 1. The list of randomizations was made using computer-generated code and a combination of 4 and 6 blocks. One of the hospital employees had no role in the study but did randomization. The outcomes were evaluated at the beginning and on the seventh day of the study.
Since a high dose of vitamin D is enough to increase the level of 25-hydroxyvitamin D to >30 mg/dL in three to five days after administration, the vitamin D group received a single dose of 300,000 IU vitamin D by intramuscular injection (11, 12). The placebo group received the same dose of an intramuscular placebo injection with no active ingredient, vitamin D. Both formulations were produced by DarouPakhsh Company, Tehran, Iran, and labeled by one of the employees not participating in the study. Patients and investigators were blinded to the allocated intervention until the final analysis.
3.5. Statistical Analysis
Based on the previous study, the required sample size was 15, but increased to 17 individuals, considering a 10% attrition rate (13). A larger sample size was included in the study to improve the accuracy of the results. For this purpose, 100 people (n = 50 in each group) were included in the study.
The data were scrutinized using SPSS software (version 26). The normality of the data was determined based on the Kolmogorov-Smirnov test. Patients' characteristics were reported using descriptive statistics. Quantitative variables between groups were compared using the T-test, while qualitative variables were compared using the chi-square and Fisher exact tests (with frequencies of <5). Differences in age, body mass index (BMI), biochemical indices, and the interval between the onset of symptoms and hospitalization were evaluated with a t-test. Mann-Whitney U test was used to compare SpO2 and respiratory rate between the two groups. The mortality and intubation rate was determined using logistic regression, and Cox regression was used for the length of stay in the hospital and ICU. The effect of confounding factors, such as age, gender, BMI, and underlying diseases, were adjusted. A test was performed to evaluate proportional hazard. A P-value of < 0.05 was considered significant.
4. Results
4.1. Patients
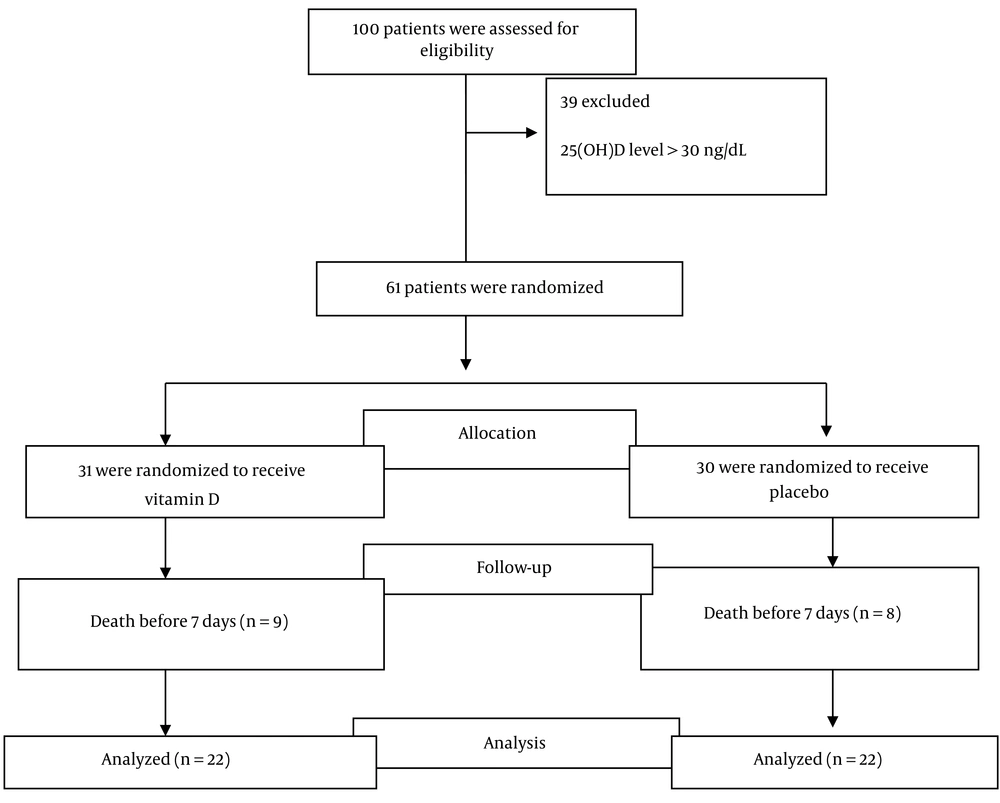
Out of 100 evaluated patients, 61 met the inclusion criteria in the study and were randomly assigned to the vitamin D or placebo groups. This trial was completed with 44 COVID-19 patients (22 patients in the intervention and 22 in the placebo groups) (Figure 1). Nine patients in the intervention group and eight patients in the placebo group died due to COVID-19 infection before seven days. The majority of patients were male (70.5%), and the mean (SD) scores of patients' age and BMI were obtained at 53.49 (13.28) years and 29.80 (5.09) kg/m2. The patients had common clinical symptoms of sore throat, shortness of breath, and cough and had, on average, eight days passed from the onset of their symptoms, were hospitalized in the ICU, and needed oxygen therapy from the very beginning (34 cases received a reservoir bag and 10 cases received noninvasive ventilation). The baseline characteristics of the two groups are presented in Table 1.
| Characteristics | Vitamin D3 Group (n = 22) | Placebo Group (n = 22) |
|---|---|---|
| Age, y | 56.33 ± 14.6 | 50.65 ± 11.4 |
| Gender | ||
| Female | 5 ± 22.7 | 8 ± 36.3 |
| Male | 17 ± 77.2 | 14 ± 63.6 |
| Body mass index, kg/m2 | 28.72 ± 5.1 | 30.88 ± 4.8 |
| The interval between symptom onset and hospitalization, d | 8.33 ± 2.5 | 8.31 ± 2.7 |
| The interval between symptom onset and enrollment, d | 10.38 ± 2.6 | 10.00 ± 2.6 |
| Acute COVID-19 symptoms | ||
| Fever and shivering | 13 ± 59.0 | 17 ± 77.3 |
| Fatigue | 7 ± 31.8 | 11 ± 50.0 |
| Cough | 10 ± 45.5 | 14 ± 63.7 |
| Dyspnea | 14 ± 63.7 | 15 ± 68.2 |
| Sore throat | 22 ± 100.0 | 22 ± 100.0 |
| Coexisting diseases | ||
| Diabetes | 3 (13.6) | 0 (0.0) |
| Hypertension | 4 (18.2) | 6 (27.3) |
| Cardiovascular disease | 1 (4.5) | 1 (4.5) |
| Medication | ||
| Remdesivir | 100 | 100 |
| Anticoagulants, heparin or enoxaparin | 100 | 100 |
| Antibiotics, ranging from imipenem to ciprofloxacin | 100 | 100 |
| Methylprednisolone O2 saturation < 90% | 95.5 | 90.9 |
| Dexamethasone O2 saturation > 90% | 4.5 | 9.1 |
| T, C | 36.80 ± 0.4 | 36.70 ± 0.5 |
| PR | 97.27 ± 15.3 | 94.68 ± 15.8 |
| RR | 20.00 ± 3.5 | 20.71 ± 2.4 |
| SBP, mmHg | 119.36 ± 15.3 | 117.22 ± 14.4 |
| DBP, mmHg | 74.91 ± 9.7 | 73.04 ± 9.0 |
| SpO2 | 81.89 ± 8.43 | 75.09 ± 18.06 |
| Oxygen supplementation | ||
| Reservoir bag | 19 ± 86.4 | 15 ± 68.2 |
| Noninvasive ventilation | 3 ± 13.6 | 7 ± 31.8 |
| Laboratory values | ||
| 25-Hydroxyvitamin D, mg/dL | 23.06 ± 5.3 | 25.68 ± 3.0 |
| Alb, IU/L | 3.41 ± 0.2 | 3.44 ± 0.3 |
| Total calcium, mg/dL | 8.24 ± 1.4 | 8.39 ± 0.7 |
| Creatinine, mg/dL | 1.14 ± 0.1 | 1.15 ± 0.1 |
| BUN, mg/dL | 38.77 ± 11.1 | 37.72 ± 11.3 |
Baseline Characteristics Regarding the Effect of High-Dose Vitamin D on the Mortality and Length of Hospital Stay of COVID-19 Patients in ICU a
4.2. Primary Outcomes
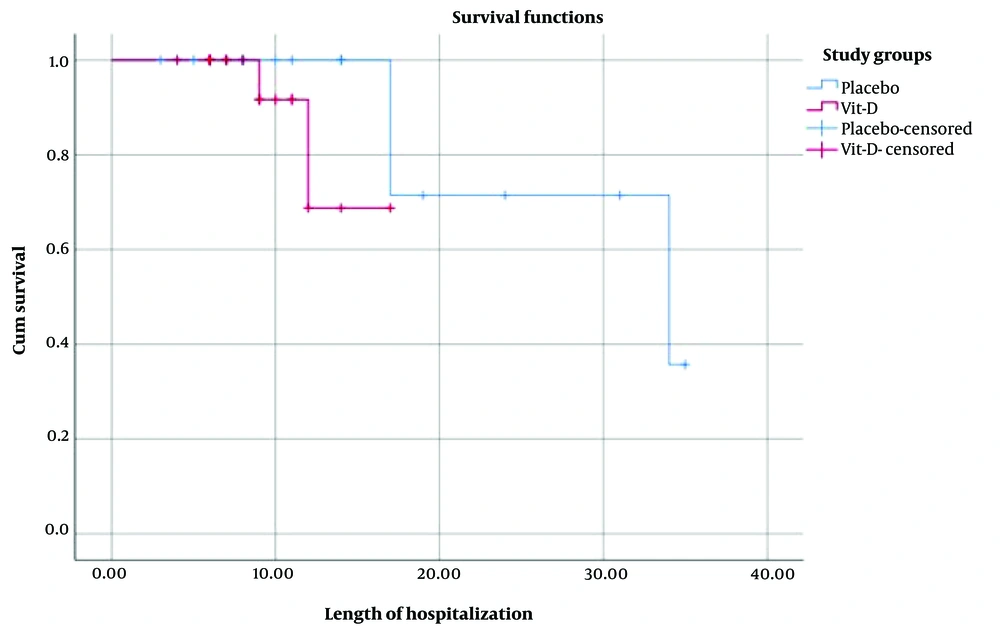
There was no significant difference in the median (interquartile range [IQR]) length of hospital stay between the vitamin D group (5.0 [6.0 - 11.0] days) and the placebo group (11.0 [6.0 - 17.0] days) (Figure 2) (Log-rank P = 0.23; unadjusted Hazard Ratio [HR] for hospital discharge, 3.4 (95% confidence interval [CI]; 0.4 - 28.05); P = 0.25; adjusted HR, 0.04 (95%CI; 0.0001 - 11.52); P = 0.27).
The chance of in-hospital mortality was 0.54 lower than in the placebo group after adjusting for confounding factors in the vitamin D group, which was not significant (Table 2) (unadjusted odds ratio (OR) for mortality,0.63 (95% CI; 0.09 - 4.21; P = 0.63), adjusted OR, 0.46 (95%CI; 0.03 - 6.55; P = 0.57).
| Outcomes | Unadjusted OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value |
|---|---|---|---|---|
| In-hospital mortality | 0.63 (0.09 - 4.21) | 0.63 | 0.46 (0.03 - 6.55) | 0.57 |
| Mechanical ventilation requirement | 2.1 (0.17 - 25.01) | 0.55 | 1.16 (0.04 - 27.57) | 0.92 |
Clinical Outcomes in a Study of the Effect of High-dose Vitamin D on the Mortality and Length of Hospital Stay of COVID-19 Patients in ICU
4.3. Secondary Outcomes
Two cases in the placebo group and one in the vitamin D group were intubated. The chance of intubation in the placebo group was 16 times higher than in the vitamin D group after adjusting for confounding factors, which was not statistically significant (Table 2) (unadjusted OR for intubation 2.1 (95%CI, 0.17 - 25.01; P = 0.55), adjusted OR, 1.16 (95%CI, 0.04 - 27.57; P = 0.92)).
Median (IQR) duration of hospitalization in ICU showed no significant difference between the vitamin D group (7.0 [4.0 - 9.0] days) and placebo group (9.5.0 [4.0 - 17.0] days) (Log-rank = 2.49, P = 0.114; unadjusted HR for ICU discharge, 6.28 [95% CI; 0.5 - 78.42]; P = 0.15; adjusted HR, 21.48 [95% CI; 0.12 - 3677.48]; P = 0.24).
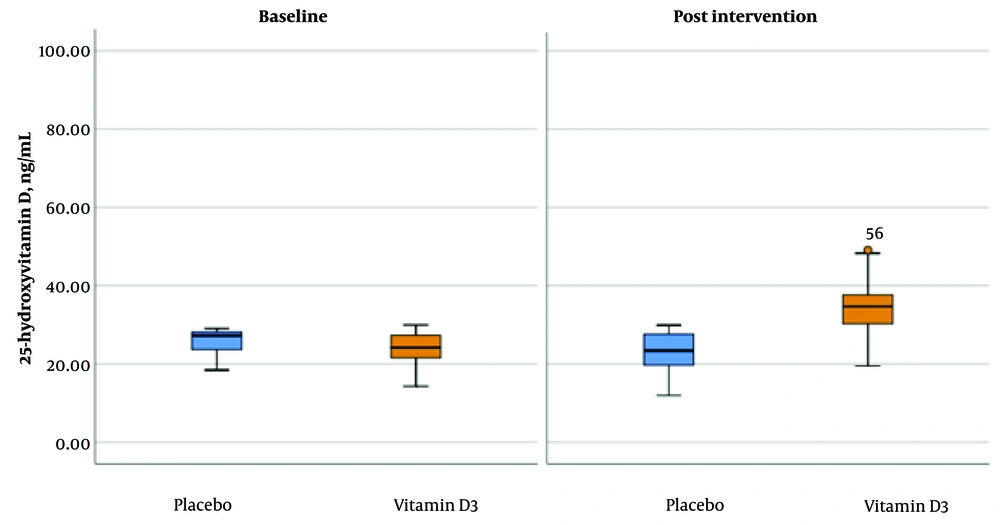
The average (SD) level of 25-hydroxyvitamin D after receiving a single high dose of vitamin D3 was significantly higher than the baseline in the vitamin D group compared to the placebo (from 23.06 (5.33) ng/mL to 34.53 (7.66) ng/mL vs from 25.68 (3.01) ng/mL to 24.00 (3.81) ng/mL in the placebo group) (Figure 3).
No violation of the proportional hazard test was observed.
5. Discussion
This placebo-controlled, double-blind, randomized clinical trial found that a single high dose of vitamin D reduced hospital stay and ICU stay in the vitamin D group; however, this difference was insignificant. In addition, receiving a high single dose of vitamin D did not significantly reduce the need for mechanical ventilation and the mortality rate in ICU patients with COVID-19.
Since vitamin D strengthens innate and adaptive immunity, it can reduce the survival and proliferation of respiratory viruses (14). In addition, the results of observational studies have demonstrated the association of higher levels of 25-hydroxyvitamin D with better clinical results in respiratory patients (15). Low levels of 25(OH) D were reported in numerous studies conducted by researchers on patients with COVID-19, which were also associated with the severity of the disease (16-18). Furthermore, there was a positive correlation between low levels of 25-hydroxyvitamin D and poor prognosis in COVID-19 patients (19). A retrospective study investigated the serum levels of vitamin D on the severity of the COVID-19 disease and its related mortality in 149 COVID-19 patients. The findings of the mentioned study revealed that the mean levels of vitamin D were significantly lower in patients with the severe-acute form of COVID-19 than in those with the moderate form of COVID-19. Serum vitamin D insufficiency (less than 30 ng/dL) was observed in 93% of people with severe-acute forms of COVID-19, which was associated with increased mortality in COVID-19 patients (20). The mean serum level of 25-hydroxyvitamin D in patients hospitalized in ICU with the severe-acute form of COVID-19 was less than 30 ng/dL.
In Murai et al., 240 patients with moderate and severe forms of COVID-19 were given a single oral dose of 200,000 IU of vitamin D3. The results showed no significant difference between the vitamin D group (n = 120) and the placebo group (n = 120) regarding the length of hospitalization and mortality (9). Based on Guven and Gultekin, receiving a single dose of 300,000 IU of vitamin D intramuscularly by COVID-19 patients hospitalized in the ICU did not decrease the length of hospitalization, mortality, and the need for intubation compared to the control group (8). Moreover, the results of a phase III clinical trial of vitamin D supplementation on 1,360 patients admitted to the ICU indicated that receiving a single dose of 540,000 IU of vitamin D enterally had no advantage over the placebo group regarding mortality (21). randomized pilot on 50 patients with COVID-19 (n = 25 in each group) receiving 0.5μg per day of calcitriol for 14 days led to a 7-fold increase in the SaO2/FIO2 ratio in the vitamin D group in comparison with the control group, which did not affect the duration of hospitalization, mortality, and intubation (10).
In the VITdAL-ICU study by Amrein, 492 patients admitted to the ICU (vitamin D3 group, n = 249; placebo group, n = 243) who were given a monthly maintenance dose of 90,000 IU vitamin D. No difference was observed in the length of stay and hospital mortality between the two groups at the end of the study (22). In Cereda et al., vitamin D supplementation was not related to hospitalization and hospital mortality, and even a 2-fold increase in mortality was observed in the vitamin D group after adjusting for confounding factors (23). In the present study, receiving a single dose of 300,000 IU vitamin D had no effect on the duration of hospitalization and mortality of patients with COVID-19 hospitalized in the ICU. The shortage of clinical benefits observed in this study did not depend on the ability of vitamin D to raise the serum level of 25-hydroxyvitamin D. In other words, patients in the vitamin D group achieved a sufficient level of 25-hydroxyvitamin D (≥ 30 ng/mL) compared to the placebo group after the intervention.
One of the reasons why vitamin D intake showed no difference between the two groups in the present study regarding mortality may be attributed to the fact that the administered vitamin D failed to find enough time to present enough activity in the body because the rapid progression of COVID-19 led to death in a short period (8). In addition, reducing circulating vitamin D binding protein during acute illness may reduce the clinical activity of cholecalciferol (24). Therefore, administering calcitriol in severe acute diseases like COVID-19 may be more effective than cholecalciferol (10). It has also been recommended that daily doses of vitamin D are more effective in treating respiratory infections than a single high dose (25). In Murai et al. (9), the interval between vitamin D administration and the onset of symptoms was 10.3 days, which agreed with that in the present study (i.e., 10.38 days). This interval can reduce the beneficial effects of vitamin D compared to the use of vitamin D in the early stages of the disease (11).
Vitamin D supplementation was ineffective in critical patients due to the development of severe acute respiratory distress syndrome (ARDS). Vitamin D supplementation before the onset of COVID-19 or in the mild stages of the disease and the development of ARDS may show immunomodulatory effects in such patients.
5.1. Strengths and Limitations
The present study was a well-designed, double-blind, randomized clinical trial in which numerous confounding factors, such as the patient's signs, medications, and disease symptoms, were controlled, and the patients were matched into the two groups. However, several concerns with our study require further studies addressing these limitations. First, the study's sample size was small, and only one center in Kermanshah City was investigated. Second, patients with COVID-19 often died in the ICU department, and it was tough to assess patients with similar conditions. Third, a dose of vitamin D3 was administered after a mean of 10.38 days from symptom onset to randomization. Further, the duration of the intervention was short. Therefore, significant changes could not be observed in some factors significantly. Due to these limitations, the results cannot be generalized to all patients with COVID-19.
5.2. Conclusions
Among critical patients with COVID-19, administering a single high dose of vitamin D compared to a placebo did not significantly reduce hospital length of stay and in-hospital mortality. The findings did not support the immunomodulatory effects of vitamin D3 in critical patients with COVID-19.