1. Background
The cesarean section is one of the most common surgeries among women (1), for which the best anesthetic choice is spinal anesthesia (2). Local anesthetic agents, the most efficient drugs for this method, potentially have undesirable side effects despite ground-breaking advances in pharmacology in recent decades. Therefore, agents are recommended to use the most efficient method with negligible side effects.
Bupivacaine (brand name Marcaine) is the preferred drug for spinal anesthesia during cesarean delivery. However, in certain situations, such as sanctions, ropivacaine 0.5% may be the only available option. Several studies have been conducted regarding the usage of ropivacaine in cesarean delivery, albeit with different concentrations or in combination with other agents.
Ropivacaine, an S-enantiomer to bupivacaine, is a long-acting amide local anesthetic with fewer cardiotoxic and neurotoxic effects (3), which was introduced and later approved by the FDA in 1996. The potency of this drug is approximately two-thirds that of bupivacaine for sensory block and half for motor block since ropivacaine’s effect on the blockage of alpha neural fibers is more significant compared to motor fibers (3).
Various surgical procedures have established preferred doses for ropivacaine, but determining the optimal dose for ropivacaine 0.5% during cesarean procedures has not been conducted since. Most dose-finding studies have used 1% and 0.75% concentrations. Choosing a safe and reliable anesthesia method with appropriate dosage can ensure maternal and infant safety after cesarean section.
2. Objectives
This study aimed to determine the appropriate dose of isobaric ropivacaine 0.5% for cesarean section and provide a basis for future research.
3. Methods
This clinical trial featured a randomized study group of 108 expectant mothers undergoing elective cesarean section. The participants, aged 18 to 42, were divided into three groups of 36 individuals each. They had attained a full-term gestational age and a height between 155 and 175 cm, with a weight range of 60 to 90 kg. These women were selected through an available sampling method and recruited from the obstetrics and gynecology department of Mo'atazedi Hospital, provided they met the inclusion criteria.
Patients with coagulation problems, increased intracranial pressure, infection at the needle insertion site, sensitivity to local anesthetics, dissatisfaction with spinal anesthesia, or study participation were excluded.
After providing a clear explanation and obtaining consent, individuals who met the eligibility criteria were recruited to participate in the study.
Patients were assured that any details they shared would remain confidential and that no personal risk would be involved. Taking part in the process was voluntary and necessitated the patient's consent. The group was chosen at random.
The following variables were recorded: Systolic and diastolic blood pressure, heart rate, arterial oxygen saturation, sensory block level (pinprick test), sympathetic block level (cold test), interval from block to maximum movement (Modified Bromage Scale), time of surgery start, prevalence of nausea and vomiting, use of vasopressors and atropine, use of auxiliary drugs such as opioids and ketamine, and block failure.
At the beginning of the procedure, 10 cc per kg of crystalloid fluid would be administered, and systolic and diastolic blood pressure, heart rate, and oxygen saturation would then be measured.
The patient was seated while the bed was placed horizontally. The space between the second and third vertebrae or between the third and fourth lumbar vertebrae was chosen for all patients. A sterile condition was maintained to achieve sensory-motor block, and a Spinal Needle Quinke No. 25 was used. Depending on the target group, the patient was injected with the spinal needle at a rate of approximately 0.2 mL/s, following the protocol: Group (1) 20 mg of 0.5% isobaric ropivacaine from MOLTENI company; group (2) 22.5 mg of 0.5% isobaric ropivacaine from MOLTENI company; group (3) 25 mg of 0.5% isobaric ropivacaine from MOLTENI company.
A vital sign was measured every five minutes after the patient lay down throughout the operation and recovery. Throughout the procedure, the patients would receive 4 - 5 liters of oxygen per minute, administered through a nasal cannula or face mask. Atropine would be injected when the patient's heart rate dropped below 60 beats per minute. Moreover, when the patient's systolic blood pressure dropped below 100 mmHg before fetal expulsion, fell below 90 mmHg after fetal expulsion, or decreased by more than 30% of the baseline, vasopressor drugs would be administered.
The patients were prescribed 1 - 2 cc sufentanil or 10 - 20 mg ketamine for mild discomfort and pain. Anesthesia was given to patients with moderate to severe pain and sensory levels below T4 when a block failed. All such cases were recorded.
After surgery, patients were monitored in the recovery room until the spinal block regressed below dermatome T10, and it was ensured that the anesthesia caused no hemodynamic changes. Patients’ satisfaction with the spinal anesthesia was recorded on a scale of excellent, good, moderate, and bad. The data were analyzed using SPSS software.
In this study, women who were candidates for cesarean section were randomly divided into three groups. Based on the following formula, the minimum sample size in each group was 36, for a total of 108 people:
Inclusion criteria: Candidates eligible for cesarean section, 18 - 42 years old, 60 - 90 kg, 155 - 175 cm height, and gestational age ≥ 36 weeks.
Exclusion criteria: History of coagulation issues, increased intracranial pressure, needle insertion infection, local anesthetic sensitivity, spinal anesthesia dissatisfaction, and reluctance to participate in the study.
4. Results
The study was conducted on 108 elective cesarean section patients in Motazedi Hospital, who were divided into 36 groups. According to the ANOVA test, there was no significant difference between the three groups in terms of age (P = 0.098), weight (P = 0.303), and height (P = 0.33).
Table 1 shows no significant correlation between age, weight, or height and the therapeutic doses studied.
| Variables | Values |
|---|---|
| Height | 162.90 ± 4.771 |
| Weight | 78.67 ± 7.713 |
| Age | 30.08 ± 5.404 |
In this study, the first group, which received a dosage of 20 mg, had the highest incidence of block failures. Four individuals experienced this outcome in said group. In the second group, only one block failure was observed. However, all attempts were successful for the third group, which received 25 mg.
Based on Table 2, the first group (20 mg) had the highest frequency of supplemental medication for optimal anesthesia, with nine cases. The second group (22.5 mg) had the lowest number of patients who required supplemental medication, with only two cases; in the third group (25 mg), there were three cases. The chi-square test indicated a significant relationship between the use of supplemental drugs and the therapeutic doses of the study groups, with a P-value of 0.016.
| Variables | Groups | P-Value | ||
|---|---|---|---|---|
| 20 mg | 22.5 mg | 25 mg | ||
| Drug usage | 0.016 | |||
| Yes | 9 (28.12%) | 2 (5.71%) | 3 (8.33%) | |
| No | 23 (71.87%) | 33 (94.28%) | 33 (91.66%) | |
Table 3 shows no significant correlation between the study groups regarding average systolic blood pressure 1 to 20 minutes after the block.
| Variable | Systolic Pressure (Mean ± SD) | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Min | ||||
| 1 | 113.37 ± 12.509 | 110.31 ± 11.720 | 103.6 ± 11.5 | 0.1 |
| 5 | 126.58 ± 20.659 | 125.3 ± 19.7 | 125.5 ± 20.3 | 0.51 |
| 10 | 127.58 ± 20.760 | 127.3 ± 20.9 | 123.2 ± 14.5 | 0.12 |
| 15 | 124.5 ± 15.7 | 123.31 ± 14.5 | 123.1 ± 13.3 | 0.08 |
| 20 | 122.50 ± 19.650 | 121.8 ± 14.3 | 121.2 ± 13.5 | 0.87 |
Based on the data presented in Table 4, it can be concluded that there is no significant statistical difference among the groups concerning the average diastolic blood pressure readings taken between the first and twentieth minute after the block. In other words, the three doses of the drug did not have a varying effect on the diastolic blood pressure.
| Variable | Diastolic Pressure (Mean ± SD) | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Min | ||||
| 1 | 79.65 ± 12.150 | 78.1 ± 12.4 | 79.6 ± 12.8 | 0.123 |
| 5 | 83.20 ± 15.311 | 80.1 ± 10 | 83.8 ± 14.30 | 0.53 |
| 10 | 81.72 ± 14.720 | 81.8 ± 10.7 | 81.3 ± 13.10 | 0.49 |
| 15 | 79.50 ± 13.50 | 78.2 ± 11.8 | 78.64 ± 12.1 | 0.45 |
| 20 | 80.5 ± 12.3 | 79.1 ± 12.75 | 77.3 ± 11.2 | 0.63 |
In addition, there was no significant statistical difference between the groups regarding the average heart rate from 1 to 20 minutes after the block. Therefore, three doses of the drug did not affect heart rate differently. Consequently, hemodynamic changes in the three doses used were insignificant, and increasing the drug dose did not lead to more hemodynamic instability in the patient (Table 5).
| Variable | Heart Rate (Mean ± SD) | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Min | ||||
| 1 | 86.65 ± 19.002 | 86.3 ± 18.4 | 85.40 ± 19 | 0.465 |
| 5 | 89.30 ± 19.625 | 87.1 ± 17.8 | 87.23 ± 18.6 | 0.18 |
| 10 | 82.5 ± 17.50 | 81.9 ± 13.8 | 81.7 ± 13.1 | 0.28 |
| 15 | 86.10 ± 18.110 | 84.7 ± 17.87 | 86.2 ± 17.32 | 0.09 |
| 20 | 87.35 ± 19.1 | 85.40 ± 19 | 85.8 ± 16.2 | 0.165 |
As shown in Table 6, the lowest frequency of vasopressor use in the first treatment group (20 mg) was 8, and the frequency of vasopressor use was equal in the second and third groups. According to the chi-square test, there was no significant relationship between the variable in question and the therapeutic doses of the study groups (P = 0.963).
| Variable | Groups | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Atropine | 0.963 | |||
| Yes | 8 (25%) | 9 (34.6%) | 9 (33.33%) | |
| No | 24 (75%) | 26 (58.38%) | 27 (66.66%) | |
As shown in Table 7, the first treatment group (20 mg) had the lowest frequency of atropine use (nine cases), while the third group had the highest frequency (11 cases). The chi-square test indicated no significant relationship between the variable in question and the therapeutic doses of the study groups, with a P-value of 0.982.
| Variable | Groups | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Vasopressor | 0.982 | |||
| Yes | 9 (29%) | 10 (28.6%) | 11 (30.6%) | |
| No | 22 (71%) | 25 (71.4%) | 25 (69.4%) | |
According to Table 8, the lowest nausea rate was observed in the first treatment group (20 mg), at 12 (37.5%). There was no significant relationship between the nausea variable and the therapeutic doses of the study groups.
| Variable | Groups | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Nausea | 0.344 | |||
| Yes | 12 (37.55%) | 19 (54.3%) | 18 (50%) | |
| No | 20 (62.5%) | 16 (45.7%) | 18 (50%) | |
This study had the lowest vomiting rate in the second treatment group (1 or 2.8%). There was no significant relationship between vomiting and the therapeutic doses of the study groups. (Table 9).
| Variable | Groups | P-Value | ||
|---|---|---|---|---|
| 20 | 22.5 | 25 | ||
| Vomiting | 0.2 | |||
| Yes | 4 (12.55%) | 1 (2.8%) | 3 (8.3%) | |
| No | 28 (87.5%) | 34 (97.1%) | 33 (91.6%) | |
The highest level of sensory block in all three groups was at the T3 and T4 dermatome levels. The highest frequency was achieved in reaching the highest level of the sensory block (area T1) in the third group (8.3%), and the highest frequency of sympathetic block levels in all groups was between T2-T3 levels (90.2%).
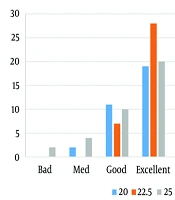
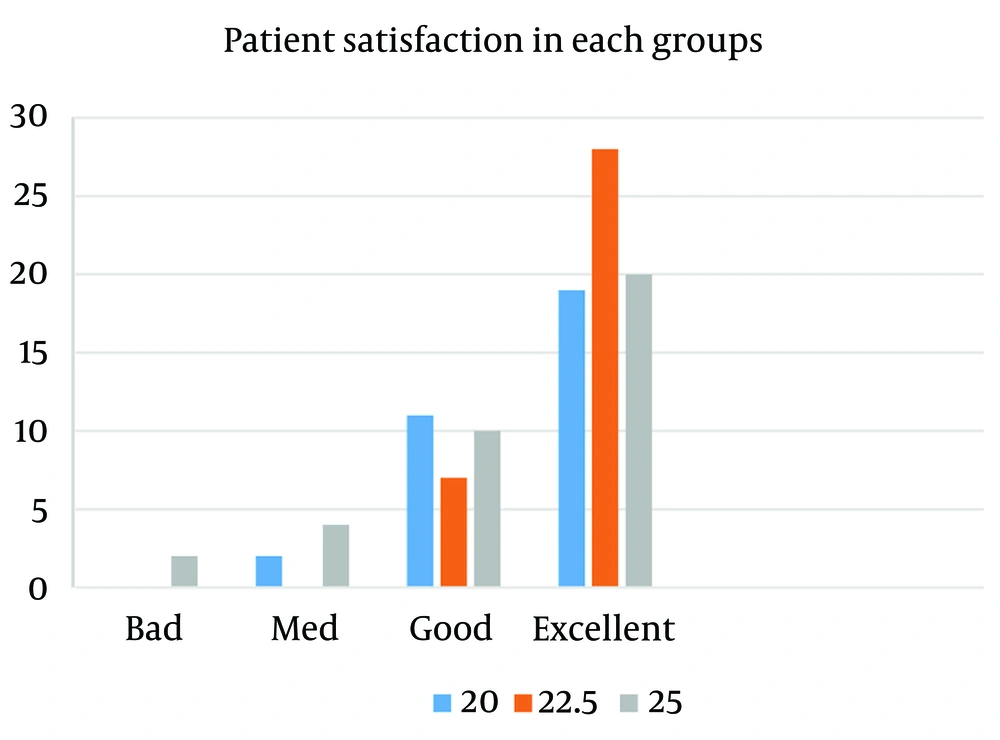
The Kruskal-Wallis nonparametric test was utilized to compare the interval between drug administration and the entire block, which did not show a significant statistical difference between the three groups (P = 0.421). In most cases, an entire motor block was obtained and performed within two minutes after spinal anesthesia. The second group observed the highest level of anesthesia satisfaction (Figure 1).
5. Discussion
Using regional anesthesia has helped avoid certain general anesthesia complications, such as intubation complications and aspiration, and the side effects of general anesthesia agents. Among local anesthesia techniques, spinal anesthesia is the most commonly used method in cesarean delivery due to its significant analgesic effect (4-6). In this method, local anesthetics can be used individually or in combination with other drugs, such as opioids.
In this study, ropivacaine 0.5% was used, which is sometimes the only available option for cesarian delivery. The greater the dose, the better the sensory block, but the more adverse side effects are likely to occur. In this study, however, the difference in the incidence of side effects between the three doses, including hypotension, bradycardia, nausea, and vomiting, was insignificant. Likewise, insignificant differences were found concerning atropine or vasopressor dosages for treating the side effects and the interval between drug administration and complete motor block. Nevertheless, incomplete anesthesia and the need for supplemental drugs in the first group indicate that 20mg was insufficient for this study. Increasing the dosage to 22.5 or 25 mg could guarantee a more effective analgesic effect without significantly increasing side effects.
According to the anesthesiology in Miller (3), the recommended dosage of ropivacaine for achieving an optimal level of anesthesia during a cesarean section (T4 level) is between 18 and 25 mg. Although the book did not specify the preferred baricity of the drug, a concentration of 0.5% to 1% can effectively determine the height of the block, even though it is a less significant factor.
Previous studies have frequently used higher concentrations of hyperbaric ropivacaine. In 2019, a dose-finding study was conducted by Zhu et al. on 500 cesarean deliveries using hyperbaric ropivacaine (Ropivacaine 0.75% + 0.5 mL of 10% glucose) with doses 10, 12, 14, and 16. The dose of 14 mg produces the best result with minimal side effects. This article used a different baricity and concentration compared to the present study and a different ideal sensory level (T6). The fact that the patients’ heights have not been mentioned in this study could also be necessary (7).
In another study, Srividya et al. compared the efficacy of isobaric ropivacaine 0.5% (18 mg) to Marcaine 0.5% in cesarean delivery and concluded that ropivacaine is an effective agent with negligible infant side effects (8).
In another study by Wang et al., they compared ropivacaine 0.75% (15 mg) and Marcaine and identified ropivacaine as an effective drug with fewer side effects (9).
In some articles, in addition to the different concentrations of the drug, ropivacaine was used in combination with opioids. This combination can help reduce the dose of spinal agents. For example, Huang et al. used ropivacaine 1% (15 mg) in combination with fentanyl and concluded that combining this drug with fentanyl reduced complications (10).
In a similar study conducted in Germany, ropivacaine (15 mg) was used alone and in combination with morphine (11). The study suggested that further research was needed to explore other combinations of doses. Another research used the same combination and found the need for further research with varying doses.
In some studies, ropivacaine has been used in combination with narcotics or used in non-cesarean surgeries (12-15). Other studies have investigated the use of this drug with epidural anesthesia (16).
Currently, there is a shortage of research on the ideal dosages of isobaric ropivacaine 0.5% for administering cesarean sections. Consequently, selecting the appropriate dose poses certain difficulties. In some cases, specific medications may be the sole viable alternative.
5.1. Conclusions
This study compared factors between three groups, including hemodynamic parameters, anesthesia complications, sensory and sympathetic block levels, injection duration to complete motor block, failure rate, and the need for supplementary drug administration. The hemodynamic changes observed in the three groups were not found to be significantly different. Therefore, the increase in the drug dosage did not cause more hemodynamic instability in the patients.
None of the three doses used was preferable to the others regarding sensory and sympathetic block levels, the time taken to reach the entire motor block, and the incidence of nausea and vomiting. However, higher doses were associated with a lower failure rate of block and supplemental drug use, and they achieved a more sufficient and satisfactory level of analgesia.
Upon careful evaluation of the results and the level of patient satisfaction, administering 22.5 mg of isobaric ropivacaine 0.5% as spinal anesthesia for cesarean section surgery is a productive approach that presents minimal risks and complications.
Developing a thorough strategy to determine the correct dosage of medication can minimize potential complications during cesarean section procedures. The effective execution of this research can potentially reduce the requirement for supplementary anesthetic drugs and treat their associated adverse reactions, creating opportunities for further investigation.