1. Background
Fatty liver is a significant cause of death related to liver diseases. Consumption of alcohol, malnutrition, fatty foods, excess weight, inactivity, high blood pressure, pregnancy, and using drugs like tetracycline and aspirin are the causes of this disease (1). Three out of ten Iranians suffer from fatty liver, with increasing prevalence in children (2). Obesity is closely linked to fatty liver. Insulin resistance, a precursor to diabetes and the basis of metabolic syndrome can harm liver cells even before diabetes onset. Metabolic syndrome, comprising high blood pressure, high blood fat, obesity, and diabetes, increases fatty liver severity (3). Most patients are asymptomatic, with the disease often detected by elevated liver enzymes in blood tests or during abdominal ultrasounds. Rarely, some may experience fatigue or vague upper right abdominal pain (4).
Most patients with fatty liver disease are diagnosed with simple liver tests in blood specimens or using imaging techniques like liver sonography (5). Increased liver enzymes, found in 50% of fatty liver cases, do not directly correlate with disease severity but reach 80% in advanced stages (6). Sonography, the most common diagnostic method, is non-invasive, affordable, and accessible. CT scans, which are more expensive and use X-rays, are less sensitive than sonography for identifying fatty liver (7). Liver biopsy is the most accurate method for evaluating liver damage in patients with suspicious history or clinical examination (8).
Vibration-controlled transient elastography offers a reliable and cost-effective alternative to liver biopsy for assessing liver stiffness, utilizing a 50Hz elastic wave detector and transducer for measurement (9). In chronic hepatitis C patients, serum levels are inversely correlated with liver fibrosis progression (10). Similarly, low serum zinc levels were significantly associated with advanced liver fibrosis in another study of chronic hepatitis C cases (11).
Accurate fatty liver and hepatic inflammation diagnosis are vital for prognosis and treatment planning (12). Early detection enhances treatment response. Ultrasound is valuable for diagnosis and monitoring, often paired with s (13).
2. Objectives
This study aims to correlate liver fibroscan results with elastography and liver enzymes in fatty liver patients, recognizing the significance of timely diagnosis.
3. Methods
This descriptive and analytical cross-sectional study included patients diagnosed with fatty liver by Fibroscan and sonography, who attended the Fatty Liver Clinic of Shahid Motahari, Shiraz University of Medical Sciences, in the first half of 2023. The inclusion criteria were fatty liver diagnosed by a gastroenterologist, and patients with liver mass or malignancy were excluded.
Data were collected using a prepared checklist, including demographic information, like age, findings from the Fibroscan, liver elastography findings, and laboratory findings, such as liver enzymes.
The data collection permission was secured from the Research Vice-Chancellor of Shiraz University of Medical Sciences after obtaining approval for the research project. Eligible patients at the Fatty Liver Clinic of Shahid Motahari in Shiraz underwent an examination by a gastroenterologist. Liver enzyme tests, Fibroscan, and liver elastography were conducted, and the results were recorded on a checklist. AST was included in the liver enzyme tests, and the CAP score was utilized to determine steatosis. A radiology assistant performed liver elastography under the supervision of a radiologist, with the Hepatorenal Index measured to determine steatosis.
3.1. Statistical Analysis
The data were analyzed using SPSS software version 24, with descriptive statistics presented in Tables and graphs showing mean and standard deviation. Chi-square and independent t-tests were used for analysis. Spearman’s correlation coefficient was employed to assess the correlation between elastography, fibroscan findings, and liver enzymes. ROC curve analysis was performed to determine the sensitivity and specificity of fibroscan, elastography, and liver enzymes in diagnosing fatty liver. The significance level was set at 0.05.
4. Results
This study aimed to correlate liver elastography results with fibroscan and liver enzymes in fatty liver patients. Based on ultrasound results, 80 patients diagnosed with fatty liver were evaluated. The mean age of the patients was 50.91 ± 9.48 years, with the oldest being 66 and the youngest 29. Among the 80 patients studied, 36 (45%) were men and 44 (55%) were women.
4.1. Ultrasound Findings
25% had grade 1, 47.5% had grade 2, and 27.5% had grade Three fatty liver. Of those diagnosed with fatty liver by ultrasound, 78.8% were confirmed by elastography (true positive), while 21.3% were falsely diagnosed as healthy.
The average age of fatty liver patients was lower than that of healthy individuals but not significantly different. Female frequency was higher but not significant. Average CAP score was higher in fatty liver patients but not considerably. The steatosis (a term healthcare providers use to describe fat buildup in an organ (usually your liver)) percentage was lower in fatty liver patients. Average AST and ALT enzymes were significantly higher in fatty liver patients than healthy individuals (Table 1).
| Variables | HRI < 1.2 b | HRI > 1.2 | P-Value |
|---|---|---|---|
| Age | 52.83 ± 9.17 | 50.44 ± 9.58 | 0.44 |
| Gender | 0.72 | ||
| Male | 7 (41.2) | 29 (46.0) | |
| Female | 10 (58.8) | 34 (54.0) | |
| CAP score | 340 ± 37.66 | 340.04 ± 40.21 | 0.99 |
| Steatosis percent | 80.76 ± 14.25 | 81.20 ± 14.84 | 0.91 |
| Steatosis stage | 0.94 | ||
| Stage II | 2 (11.8) | 7 (11.1) | |
| Stage III | 15 (88.2) | 56 (88.9) | |
| AST | 26.47 ± 3.62 | 32.08 ± 21.24 | 0.045 |
| ALT | 29.05 ± 10.43 | 47.88 ± 39.43 | 0.001 |
Distribution of Study Variables Between Patients Diagnosed with Fatty Liver and Healthyin Elastography a
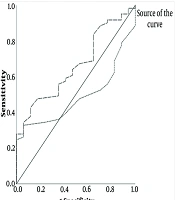
A positive but weak correlation between Ratio and CAP score was found (Figure 1), which was insignificant (r = 0.018, P = 0.87). ROC curve analysis showed that the cutoff point values of 320 for the CAP score, with AUC = 0.500, sensitivity 66.7%, and specificity 70.6%, which were not statistically significant (P = 0.99). Thus, the The CAP score from a fibroscan cannot predict fatty liver when diagnosed by elastography.
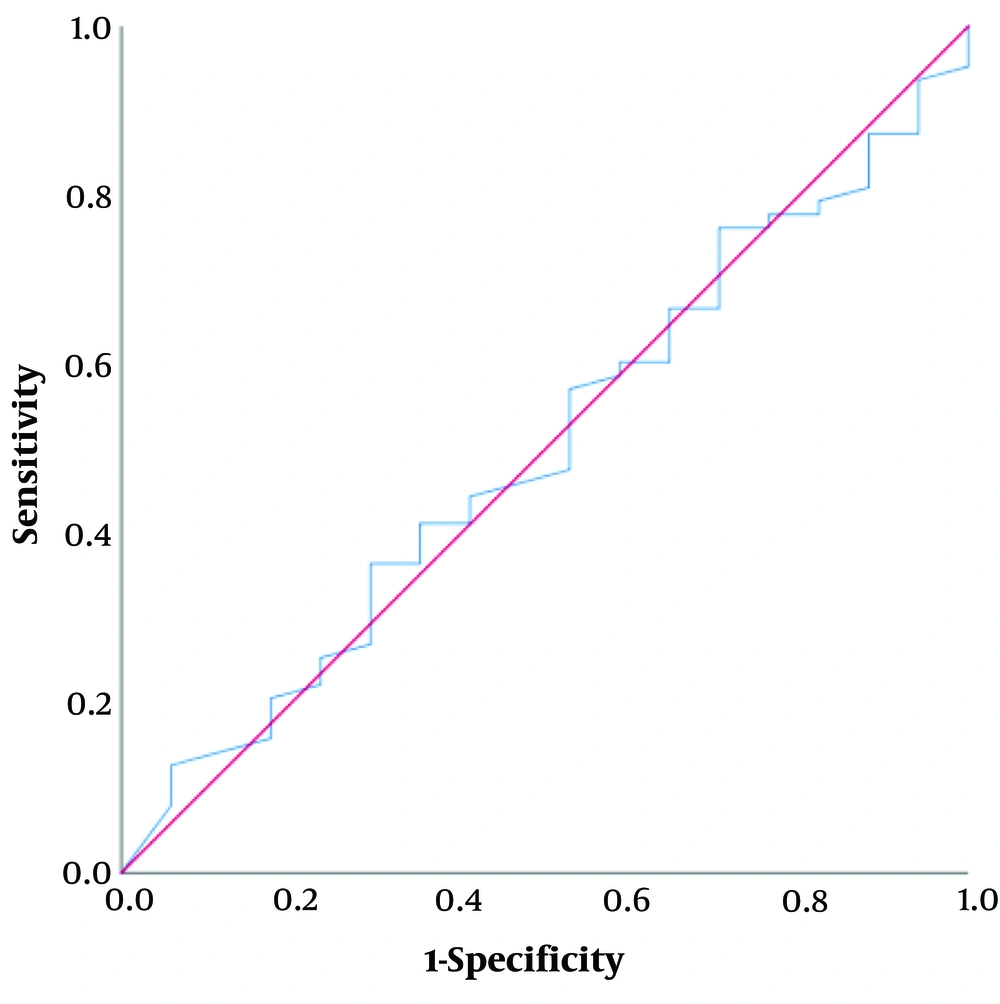
A positive but weak correlation between Ratio and AST was not significant (r = 0.024, P = 0.83). ROC curve analysis (Figure 2) for AST showed nonsignificant results (P = 0.85), indicating AST cannot predict fatty liver.
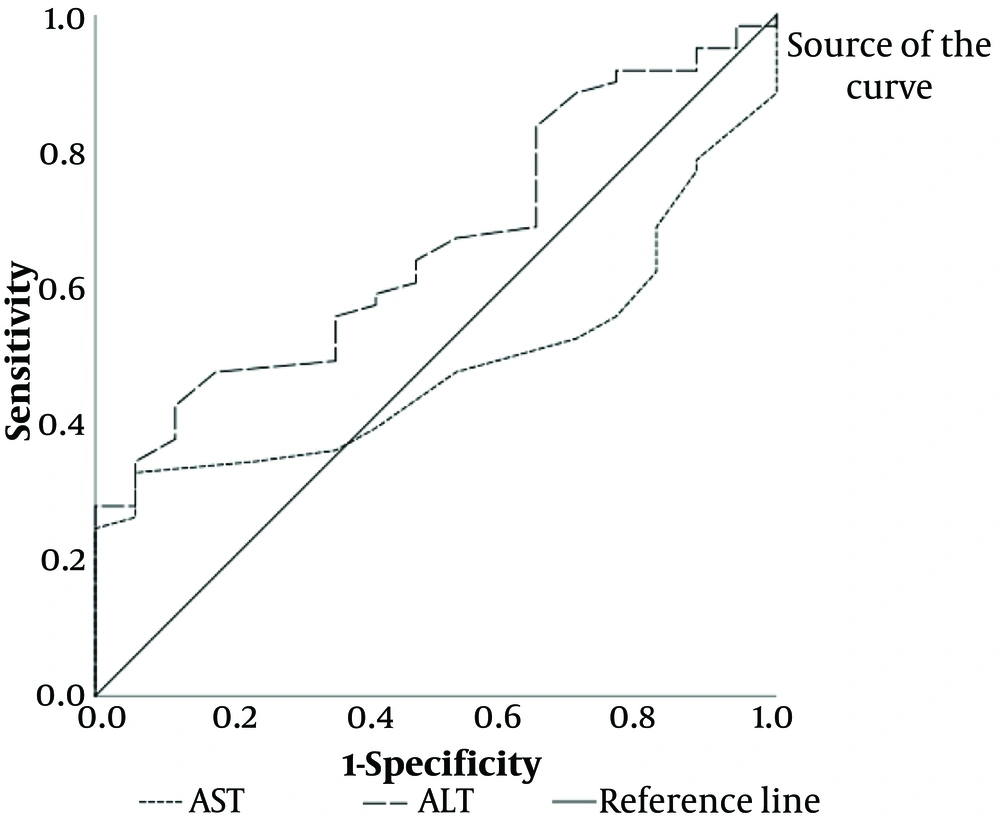
There was a positive but weak correlation between Ratio and ALT (r = 0.173, P = 0.13). A ROC curve analysis for ALT revealed significant results (P = 0.043), indicating that ALT is 82.6% sensitive and 65% specific for predicting fatty liver.
5. Discussion
In non-alcoholic patients, hepatic steatosis poses a significant public health risk. Non-alcoholic fatty liver disease (NAFLD) accounts for 75% of the chronic liver disease burden in the Western world, leading to severe complications and often requiring liver transplantation (14, 15). Diagnosis typically involves routine ultrasound and liver enzyme tests, with liver biopsy as the gold standard. Non-invasive imaging methods like ultrasound, CT, and MRI are effective but may not distinguish between types of liver fat (16-19). Transient elastography, particularly the CAP parameter, offers a quantitative assessment of liver fat but is operator-dependent (20). Given steatosis’s significance in NAFLD, accurate evaluation is crucial for treatment. Ultrasound remains the most cost-effective and common method for diagnosing liver steatosis, characterized by specific sonographic findings (21).
Ultrasound lacks objectivity in measuring liver steatosis (17). Elastography, including point shear wave elastography (pSWE) and two-dimensional shear wave elastography (2D-SWE) offers a new dimension to liver assessment (22, 23). Unlike transient elastography (TE), 2D-SWE is unaffected by obesity and ascites (24). However, the exact role of elastography in diagnosing fatty liver disease is still unclear.
The present study aimed to correlate liver elastography results with fibroscan and liver enzymes in fatty liver patients.
Initial research on the Hepatorenal Index (HRI) has indicated strong diagnostic precision (17, 25, 26). Stahlschmidt et al. (27) confirmed its effectiveness in identifying various levels of steatosis, echoing the present results. Previous studies have also endorsed HRI’s utility in diagnosing fatty liver disease without advanced fibrosis (28). Johnson et al. (15) highlighted its 72% accuracy in classifying hepatic steatosis, underscoring its value in monitoring disease evolution and treatment efficacy. However, Kjaergaard et al. (29) and Moret et al. (30) reported moderate diagnostic accuracy in alcoholic and non-alcoholic fatty liver disease cases. Moret et al. found similar results in detecting any degree of steatosis (≥ S1) using HRI through B-mode ultrasound (30). Yet, the present study surpassed these findings in accuracy (31, 32).
The variance between the present study and these previous ones could be attributed to image quality discrepancies. A prior study assessed high-quality and low-quality images, unlike ours, which only evaluated high-quality images. Kjaergaard et al. (29) noted that diagnostic accuracy improved from moderate to good when only high-quality images were considered, although this enhancement was not statistically significant. This underscores the significance of utilizing high-quality measurements.
Marshall et al. discovered that the Hepatorenal Index (HRI) effectively excludes hepatic steatosis, reducing unnecessary biopsies (33). Similarly, Shiralkar et al. showed that HRI, determined via DICOM images in PACS without extra software, detects steatosis above 5%, making it cost-effective for liver evaluation (34). Quantifying steatosis with HRI aids treatment assessment and monitors therapeutic responses, especially in treated patients (15). However, HRI cannot grade steatosis in advanced chronic liver disease, chronic kidney disease, absence of the right kidney, or liver masses near the right kidney (35).
CAP, often performed alongside transient elastography (FibroScan), is a relatively new test validated in large studies for detecting various degrees of steatosis. However, the specialized equipment used for CAP is not versatile for other imaging purposes. The present study found a very weak correlation between the hepatorenal index measured by elastography and CAP measured by liver fibroscan. Liver fibroscan results were not reliable for diagnosing fatty liver disease. Nevertheless, CAP scores alone may not be a good indicator for diagnosing fatty liver disease, especially without elastography steatosis scoring.
In this study, a weak correlation was observed between the CAP score and Ratio B-Mode. Kjaergaard et al. (29) found that Ratio B-Mode with CAP score was comparable in diagnostic accuracy, but it was highly susceptible to failure, limiting its clinical utility. A CAP is a semi-quantitative technique that is independent of the machine and operator and less affected by sampling error than a liver biopsy since it examines a much larger volume of liver (36). However, there is limited research on CAP is present in chronic liver disease patients and none in the general population.
Additionally, CAP is unavailable when using the XL probe, indicating a need for further development and validation (37).
In this study, a positive correlation was observed between liver enzymes (ALT and AST) and elastography findings. However, this correlation was not statistically significant. Furthermore, our results revealed that while AST could not predict fatty liver diagnosed by elastography, ALT demonstrated predictive capability with 83.6% sensitivity and 67.4% specificity.
Consistent with this study, Ayonrinde et al. (38) found a positive association between serum ALT and liver fibrosis diagnosed by elastography. However, liver biochemistry combining ALT without AST did not significantly correlate with liver fibrosis, corroborating our results. Similarly, Rasul et al. (39) reported no significant relationship between elastography fibrosis score and AST/ALT values in NAFLD patients, aligning with our findings. Feng et al. (40) demonstrated a strong correlation between SWE and liver function tests in severe fibrosis and cirrhosis. In contrast, Yoon et al. (41) reported a significant correlation between two-dimensional SWE and AST, ALT, and triglyceride-glucose index, differing from our findings. Risk factors such as metabolic syndrome, diabetes, and obesity, alongside elevated liver enzymes, increase the likelihood of NAFLD. However, ALT alone may not be reliable in predicting NAFLD (42, 43).
According to this study, elastography accurately diagnosed 78.8% of fatty liver patients identified by ultrasound, making it a useful noninvasive tool for diagnosing fatty liver disease. A significant correlation was not found between fibroscan findings and liver enzymes and elastography results, suggesting they may not be reliable indicators of fatty liver. No single method alone is sufficient for diagnosing and staging fatty. liver disease, and employing a combination of different methods may be more beneficial.