1. Background
Systemic lupus erythematosus (SLE) is a potentially fatal type of autoimmune systemic disease with extensive clinical and immunological manifestations that affect various organs, causing severe damage to patients. The global prevalence of SLE varies widely across different populations and geographical regions, with estimates typically ranging from 20 to 150 cases per 100,000 individuals. The disease predominantly affects women of childbearing age, with a female-to-male ratio of approximately 9: 1. Although SLE can affect people of any race or ethnicity, those of African, Hispanic, or Asian heritage are more likely to experience it than Caucasians. Genetic predisposition, environmental factors, and healthcare access may influence regional differences in SLE prevalence. Two main mechanisms of autoimmunity are induced by the activation of autoreactive T cells and the overproduction of autoantibodies by B cells (1). Lupus mainly affects women, and more than 90% of lupus patients are women in their reproductive age (2).
The key disorders of lupus activated by the mechanisms mentioned above include the abnormal increase in the production of cytokines such as interferon, interleukin (IL-6, IL-17, IL-12, IL-23), and B lineage cell abnormalities (3). The results of this dysregulation in the immune system are hyperactivity of helper T cells and activation of B cells, which lead to the overproduction of antibodies and can cause tissue damage due to immune complex formation and activation of the complement cascade or cytotoxicity (4).
Antibodies produced in SLE patients stick to the cell membrane or are deposited in the joints by forming immune complexes and cause arthritis. Moreover, some antigens on the surface of red blood cells, lymphocytes, and platelets stimulate auto-antibodies and cause blood complications in SLE patients. Glomerulonephritis, skin manifestations, arthritis, cytopenia, hemolytic anemia, vasculitis, and neuropsychiatric disorders are among other major complications of SLE (5-7).
Although the remissions and exacerbations of SLE are highly unpredictable, risk factors can be avoided to reduce the incidence of flares and help reduce morbidity and mortality (8). In this regard, finding the right therapeutic option for resistant patients is essential. Treatments for lupus include hydroxychloroquine, corticosteroids, immunosuppressive drugs, and some biological drugs such as belimumab. However, these interventions are not always effective and may lead to organ damage (9). Since SLE in humans is characterized by functional abnormalities of B cells, focusing on B cell depletion may be an effective therapeutic strategy.
Rituximab is a monoclonal antibody that predominantly targets the protein CD20, which is present on the surface of B lymphocytes. Rituximab induces the depletion of B cells through mechanisms such as antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and apoptosis by binding to CD20. B cells play a crucial role in the pathogenesis of SLE by producing autoantibodies, presenting antigens, and secreting pro-inflammatory cytokines. Therefore, the depletion of B cells by rituximab is expected to reduce autoantibody production and modulate the autoimmune response, potentially leading to clinical improvements in SLE patients (10). Good therapeutic responses in the refractory manifestations of SLE patients with a variety of disease conditions have been reported due to the use of rituximab (RTX) in the literature (11, 12).
2. Objectives
However, very few studies have been conducted on the effects of RTX biosimilar (Zytax) on refractory manifestations of SLE in Iran, and most focus on nephritis complications. The therapeutic effects of RTX were investigated in 38 SLE patients with refractory manifestations who were resistant to most of the standard treatments.
3. Methods
This case series descriptive-retrospective study was conducted on SLE patients resistant to standard treatments or with vital organ damage who were referred to the Rheumatic Diseases Research Center, Mashhad University of Medical Sciences, Mashhad, Iran, from 2017 to 2022.
3.1. Inclusion and Exclusion Criteria
The study population included patients diagnosed with SLE based on Systemic Lupus International Collaborating Clinics criteria (version 2012) who received RTX biosimilar Zytax at least in the previous five years and whose medical records were available. Resistance to conventional treatment was the other inclusion criterion. Patients with incomplete medical records and those under 18 were excluded from this study.
3.2. Study Design
The medical records of all SLE patients who were resistant to treatment and underwent RTX therapy due to refractory manifestations during the last five years (2017 to 2022) were assessed, and 55 patients were selected through census sampling. Often, refractory was considered refractory to the conventional cytotoxic and glucocorticoid therapies for at least three months. Out of these 55 patients, 10 cases were excluded due to incomplete data (two patients with thrombocytopenia, five patients with nephritis, and two patients with arthritis), and seven cases due to dying at starting of RTX therapy. Eventually, the remaining 38 patients were included in this study, and the data were gathered from patients' medical records. A researcher-made checklist was used to collect data such as demographic characteristics, disease duration, the reason for RTX administration, drugs used before RTX administration, laboratory examination (three times included: At the start of refractory manifestations, just before and six months after the last RTX injection), and response to RTX therapy. In most of the patients, low-dose conventional immunosuppressive treatment was continued (Methotrexate: 7.5 mg/week, Azathioprine: 50 - 100 mg/d, or Mycophenolate Mofetil: 500 - 1000 mg/d)
In quantitative variables, serum creatinine and 24-hour urine protein levels were examined in patients with renal disorders. Patients with thrombocytopenia were assessed by measuring platelet levels. Anti-DNA, C3, and C4 levels were compared in serial visits. Rituximab is a biosimilar Zytax (500 mg each vial) produced by Orchid Pharmed company in Iran. The standard dose for administration is 1g/week in two-week intervals for the first infusion (2g total) and later 1g every six months. Premeditations, including 100 mg solumedrol, one chlorpheniramine, and one cimetidine IV infusion, were administered before each Zytax infusion as per our local protocol. C3, C4, and anti-dsDNA were calculated by the measured amount of the variable divided by the standard value of laboratory as C3 ratio (C3r), C4 ratio (C4r), and anti-dsDNA ratio (antiDNAr).
3.3. Statistical Analysis
The data were analyzed in SPSS software (version 20) using the Kolmogorov–Smirnov test (to determine data normality) and Friedman and repeated measures ANOVA tests. A P-value less than 0.05 was considered significant.
3.4. Ethical Consideration
The study was approved by the Ethical Committee of Gonabad University of Medical Sciences (IR.GMU.REC.1400.144). The data were coded and recorded into checklists to maintain confidentiality.
4. Results
A total of 38 patients out of 55 who received Zytax therapy with a mean ± SD age of 37.13 ± 89.33 years were included in this study, of whom 4 (10.5%) and 34 (89.5%) were male and female, respectively. The average age of onset of rheumatic disease was 33.89 ± 13.37 years. Seven cases (two men and five women) out of 55 expired due to TTP (n = 3), alveolar hemorrhage (n = 2), and sepsis (n = 2) at the starting time of RTX therapy.
Before the RTX administration, azathioprine 31.6% (n = 12), mycophenolic acid 34.2% (n = 13), methotrexate 39.5% (n = 15), cyclophosphamide 23.7% (n = 9), and tacrolimus 13.2% (n = 5) were administered to the patients. The mean ± SD time interval between starting RTX therapy after diagnosing the disease manifestation as a refractory problem was 7.43 ± 12.33 days. In addition, 21.1% (eight cases) of the patients showed mild sensitivity to Zytax infusion, including urticaria, dyspnea, and face edema.
Table 1 presents the frequency of RTX patients and the related reasons. The most common reasons for rituximab administration were the presence of refractory nephritis (26.3%), cutaneous problems (23.7%), and arthritis manifestation (18.4%), respectively. An improvement in the symptoms of arthritis (according to the number of swollen joints and patients and physician visual analogous scale (VAS) and cutaneous complications (discoid lupus erythematosus (DLE) size or skin vasculitis remission) has been reported up to the six months after the last RTX therapy in 57.1% (n = 4) and 44.4% (n = 4) of patients, respectively. The treatment stages of some SLE patients with refractory manifestations are detailed in Table 2. Finally, one patient with cutaneous manifestation entered the study but expired during 1-year follow-up; we could not find the reason.
| Variables | No. (%) |
|---|---|
| Reason for rituximab administration | |
| Nephritis | 10 (26.3) |
| Thrombocytopenia | 4 (10.5) |
| Lupus +rheumatoid arthritis | 7 (18.4) |
| Lupus +antiphospholipid syndrome | 1 (2.6) |
| Cutaneous problem | 9 (23.7) |
| Multiple sclerosis | 2 (5.3) |
| Sjogren's syndrome | 2 (5.3) |
| Lymphomas and others | 3 (7.9) |
| Received RTX dosage | |
| Once | 18 (47.4) |
| Twice | 12 (31.6) |
| Three times | 3 (7.9) |
| Four times | 4 (10.5) |
| Five times | 1 (2.6) |
Frequency of Patients Who Received Rituximab Dosage and the Reasons for Rituximab Administration
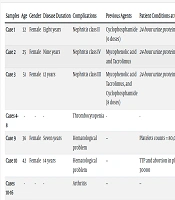
| Samples | Age | Gender | Disease Duration | Complications | Previous Agents | Patient Conditions at the Start of Manifestations | Patient Conditions Before RTX Injection | Patient Conditions After RTX Injection |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 32 | Female | Eight years | Nephritis class II | Cyclophosphamide (6 doses) | 24-hour urine protein:2000 ml | 24-hour urine protein:4000 ml | 24-hour urine protein < 500 ml |
| Case 2 | 25 | Female | Nine years | Nephritis class IV | Mycophenolic acid and Tacrolimus | 24-hour urine protein:1800 ml | 24-hour urine protein:3900 ml | 24-hour urine protein < 400 ml |
| Case 3 | 51 | Female | 12 years | Nephritis class III | Mycophenolic acid Tacrolimus, and Cyclophosphamide (8 doses) | 24-hour urine protein:2800 ml | 24-hour urine protein:5700 ml | 24-hour urine protein < 900 ml after five doses of RTX |
| Cases 4-8 | - | - | - | Thrombocytopenia | - | - | - | Increased platelet count |
| Case 9 | 36 | Female | Seven years | Hematological problem | -- | Platelets counts = 80,000 | TTP and coagulation problems | Platelet counts: 226000 |
| Case 10 | 42 | Female | 14 years | Hematological problem | -- | TTP and abortion in platelet counts achieved to 30000 | -- | Platelet count: 90000 after two doses of RTX injection |
| Cases 10-16 | - | - | - | Arthritis | -- | -- | -- | Remission rate: 57.1 |
| Cases17 | 29 | Female | Ten years | Sjogren's syndrome | -- | -- | Severe dry eyes | Improved after two doses of RTX injection |
| Case 18 | 60 | Male | 11 years | Papillary thyroid carcinoma | -- | Diagnosis of resistant lymphadenopathy in Kikuchi-Fujimoto disease | Severe dry eyes | Improved after two doses of RTX injection |
Treatment Stages of Some Systemic Lupus Erythematosus Patients with Refractory Manifestations
Table 3 compares creatinine, 24-hour urine protein collection, platelet count, and anti-DNA, C3, and C4 serum levels in lupus patients with various refractory manifestations before and six months after the last RTX administration. Based on the results, a reduction in the creatinine level was observed in lupus patients (n = 10) with nephritis after RTX injection (F = 10.42, P = 0.006). However, there was no significant difference in the 24-hour urine protein collection rate in the patients with nephritis before and after RTX therapy (F = 1.59, P = 0.23). Platelet count was not significantly different in lupus patients with thrombocytopenia (n = 4) before and six months after RTX injection (F = 6.74, P = 0.052). No significant differences in the levels of anti-DNA R (Z = -1.604, P = 0.109), C3 R (Z = 0.001, P > 0.99), and C3 R (Z = -1.06, P = 0.28) were reported after RTX administration in lupus patients with arthritis manifestation (n = 7). In addition, there was no significant difference in the levels of dsDNAr (Z = -1.09, P = 0.27), C3r (Z = -1.06, P = 0.28), and C4r (F = -1.06; P = 0.28), in lupus patients with cutaneous complication (n = 9). The mean ± SD value of C3r was 1.26 ± 0.36 and 1.16 ± 0.44 before and after RTX injection, respectively. Moreover, the mean ± SD value of C4r was 1.91 ± 1.27 and 1.91 ± 1.27 before and after RTX injection, respectively. Comparison of C3r (t = 0.63, P = 0.53) and C4r (t = 1.72, P = 0.101) before and after RTX injection showed no significant differences. The mean of anti-DNAr was 1.56 ± 1.76 and 0.87 ± 1.05 before and after RTX injection, respectively. There was, however, a significant difference before and after RTX injection in terms of anti-DNAr (Z = -2.89, P = 0.004).
| Refractory Manifestations | Test | Times | Mean ± SD | Statistical Test | P-Value |
|---|---|---|---|---|---|
| Nephritis (n = 10) | Creatinine | Start of refractory manifestations | 1.1 ± 0.47 | 10.42 b | 0.006 |
| Before RTX injection | 1.19 ± 0.46 | ||||
| Six months after the RTX injection | 0.85 ± 0.23 | ||||
| 24-hour urine protein | Start of refractory manifestations | 2131.36 ± 2365.31 | 1.59 a | 0.23 | |
| Before RTX injection | 1911.55 ± 1350.04 | ||||
| Six months after the RTX injection | 920.88 ± 939.82 | ||||
| Thrombocytopenia (n = 4) | Platelet count | Start of refractory manifestations | 92.33 ± 14.97 | 6.74 a | 0.052 |
| Before RTX injection | 68.25 ± 5 | ||||
| Six months after the RTX injection | |||||
| Arthritis manifestation (n = 7) | dsDNA | Start of refractory manifestations | 2.96 ± 2.96 | 2.62 a | 0.18 |
| Before RTX injection | 2.25 ± 1.5 | ||||
| Six months after the RTX injection | 0.76 ± 0.4 | ||||
| C3 | Start of refractory manifestations | 1.27 ± 0.46 | 0.62 a | 0.58 | |
| Before RTX injection | 1.32 ± 0.12 | ||||
| Six months after the RTX injection | 1.16 ± 0.58 | ||||
| C4 | Start of refractory manifestations | 1.62 ± 1.17 | 1.99 a | 0.25 | |
| Before RTX injection | 1.85 ± 0.71 | ||||
| Six months after the RTX injection | 1.46 ± 0.94 | ||||
| Cutaneous complication (n = 9) | dsDNA | Start of refractory manifestations | 0.87 ± 1.09 | 0.59 | 0.59 |
| Before RTX injection | 1.22 ± 0.89 | ||||
| Six months after the RTX injection | 0.78 ± 0.66 | ||||
| C3 | Start of refractory manifestations | 1.4 ± 0.42 | 2.27 a | 0.3 | |
| Before RTX injection | 1.16 ± 0.58 | ||||
| Six months after the RTX injection | 1.32 ± 0.79 | ||||
| C4 | Start of refractory manifestations | 1.3 ± 0.42 | 0.15 a | 0.86 | |
| Before RTX injection | 2.38 ± 1.83 | ||||
| Six months after the RTX injection | 1.51 ± 0.93 |
5. Discussion
The findings showed that nephritis, cutaneous, and arthritis manifestations were the patients' most common reasons for RTX administration, respectively. An improvement was reported in arthritis symptoms according to the number of swollen joints, patient and physician’s VAS score (57.1%), and cutaneous manifestation based on the DLE size or skin vasculitis remission (44.4%). A total number of eight deaths were recorded. Seven patients died at the beginning of RTX therapy due to severe emergency presentations, and one patient died after stopping the treatment in follow-up. A reduction in the creatinine level was observed in SLE patients with nephritis after RTX injection. However, there was no significant difference in the 24-hour urine protein collection rate in lupus patients with nephritis, platelet counts in patients with thrombocytopenia, and anti-DNA and C3 levels in patients with arthritis and cutaneous manifestation before and after RTX therapy. Anti-DNA was decreased in SLE patients after RTX injection, whereas C3 and C4 levels were not changed.
The management of SLE is very complicated due to unpredictable and variable organ system involvement, as well as clinical and serological presentations in various patients and within the same patient over time. Multiple parts of the body may be affected by SLE, and renal involvement is one of the most severe complications of the condition (13). A literature review showed that musculoskeletal (arthritis), renal, and hematological manifestations are SLE's most common refractory manifestations (5, 14). Similar findings were found in one study conducted on the Iranian population (15). In the present research, nephritis was the common reason for administering rituximab in SLE patients. Some degree of renal involvement has been reported in almost all patients with SLE, and between 40% and 70% of the patients develop clinically diagnosed lupus nephritis accordingly (10, 16). Five-year survival in lupus patients with renal involvement is low (17, 18). Therefore, assessing new treatment methods is crucial in controlling these side effects and their dangerous consequences. In this study, the therapeutic effects of RTX were evaluated in treating resistant SLE with various refractory manifestations.
Based on the results obtained, serum creatinine levels decreased in lupus nephritis patients after RTX injection; however, no significant difference was observed in these patients' 24-hour urine protein collection level. Systematic reviews and meta-analyses conducted on 588 patients showed that RTX increased rates of total and complete renal remission compared to the control group without increasing adverse events (14). Similarly, they reported a decrease in serum creatinine levels after RTX injection. Moreover, the researchers showed that RTX injection did not change the proteinuria level (19).
There are contradictory results regarding the effect of RTX injection on proteinuria levels. While some data emphasized the decreasing proteinuria levels in patients with lupus nephritis after RTX injection (20-22), other findings do not show a change in the amount of proteinuria after RTX treatment (14, 19). The discrepancies may be rooted in differences in drug dosage, treatment duration, patient condition, or other intervening variables. Further studies are recommended in this regard.
In another study, no significant changes were observed in creatinine clearance and urine protein parameters of patients with nephritis despite improvement in most patients and reduction of disease activity after RTX therapy (23). In a recent study by Rovin, RTX has decreased CD19+ B cells in patients with lupus nephritis, depleting peripheral CD19+ B cells in 71 of 72 patients. RTX patients reported A significant remission rate in anti-dsDNA, C4, and C3 levels. However, no significant difference was observed in total and complete renal remission rates between patients with lupus nephritis treated with RTX and the control group (18). Similarly, a decrease was reported in anti-DNA in SLE patients after RTX injection, but C4 and C3 levels had not changed (20). This may be related to the disease's severity or the patients' individual characteristics. The management of SLE is very complicated due to the unpredictability of the disease's clinical and serological presentations in various patients and within the same patient over time.
These contradictory results indicated that more intervening factors should probably be considered in measuring creatinine and urine protein levels in patients with lupus nephritis, and further studies are needed to reject or accept the present findings.
Moreover, no significant increase in the platelet count was reported in SLE patients with thrombocytopenia manifestation in the present study. Based on Ilizaliturri-Guerra et al., using low-dose RTX was influential in managing treatment-resistant severe thrombocytopenia in SLE patients (24). However, Cobo-Ibáñez et al. indicated that RTX had a short-term effect on thrombocytopenia in this group of patients (25). Another study showed that half of the thrombocytopenia patients who received rituximab were treated entirely with platelet counts of ≥ 150,000 /mL in the fifth week after the last injection of RTX. However, the mentioned study was conducted on patients with refractory autoimmune thrombocytopenia associated or not with SLE (26). Other studies confirmed the effect of a low dose of RTX in treating thrombocytopenia in treatment-resistant SLE patients in China. In one study, patients achieved complete responses (CRs, platelet count >100 × 109/l) after four courses of low-dose rituximab infusion (two infusions of 200 mg every two weeks) (27). In another, a remarkable increase in platelet counts (66.53 × 109/mL) was reported after one month (28).
Like other investigations, this study indicated that individuals with SLE experienced remission in their cutaneous manifestations and arthritis; nevertheless, there was a notable alteration in the dsDNA level following treatment, but not in the C3 or C4 levels. Although B-cell depletion therapy is an effective treatment option for rheumatoid arthritis, it often does not result in complete B-cell depletion. Half of the patients who initially show a complete B-cell depletion and clinical response after RTX treatment eventually lose their response with further injections. However, this is not a stable situation, and around three-quarters of patients respond to treatment in the following treatment cycle (29). These findings were confirmed in other studies (25, 30). Risselada and Kallenberg showed that intravenous rituximab therapy (1000 mg) along with methylprednisolone (100 mg) at a time interval of two weeks led to a significant and continuous improvement of cutaneous manifestations (31). Another study investigated the decreasing response of B cells due to the use of rituximab in cutaneous lupus and confirmed a complete clinical response of cutaneous manifestations of the systemic disease to rituximab in most patients with cutaneous lupus (32).
According to the present study, using rituximab to treat SLE complications can play an essential role in treating these patients and improve their quality of life by reducing the recurrence periods of this disease. However, studies that have focused on the effectiveness of RTX on different manifestations of SLE are lacking. Future clinical trial studies with a larger sample size and control group focusing on the effects of various doses of RTX on lupus complications and relapse periods with the control of intervening factors are recommended.
The inconsistent results regarding the effects of RTX injection on lupus patients may be rooted in entering the patients at different stages of organ involvement in the disease, which makes it difficult to compare studies. Moreover, the medication received before the start of RTX can also affect the response to RTX with a delayed effect. The number of drug administrations and definition of resistant manifestations of the disease (refractory organ involvement) is not the same in different studies. In addition, since the patients treated with RTX are commonly end-stage or resistant to the treatment, the possibility of patients’ death and their withdrawal from the final analysis is high. On the other hand, anti-rituximab antibodies predict infusion-related reactions among lupus patients. Although RTX is commonly well tolerated, infusion-related reactions have been introduced as one of the main adverse effects of the agent. The incidence of infusion-related reactions is higher among lupus patients compared to those with rheumatoid arthritis due to the higher formation of anti-drug antibodies, but its frequency has been estimated to be between 3.5% and 19 % (33-35). When interpreting the rituximab assessment, these issues should be considered since they can affect the treatment's results.
5.1. Strengths and Limitations
This was the first study in Iran focusing on the effectiveness of RTX biosimilar Zytax on different refractory manifestations of SLE patients. The results obtained can provide proper information on the effects of RTX (Zytax) on SLE in the Iranian population. However, the present study has limitations due to its small sample size. Moreover, the study's retrospective nature prevents us from generalizing the results to other populations.
5.2. Conclusions
The results showed that nephritis and arthritis were the most common complications of lupus, making the studied patient a candidate for rituximab. RTX could be suggested as a proper treatment in SLE patients with nephritis, arthritis, and cutaneous manifestations resistant to first-line treatment.
The findings showed that nephritis, cutaneous, and arthritis manifestations were the most common reasons for RTX administration. An improvement in arthritis symptoms and cutaneous manifestation has been reported in 57.1% and 44.4% of patients, respectively. A reduction in the creatinine level was observed in SLE patients with nephritis after RTX injection. However, there was no significant difference in the rate of 24-hour urine protein collection in lupus patients with nephritis; platelet count in patients with thrombocytopenia, and anti-DNA, C3, and C3 levels in lupus patients with arthritis and cutaneous manifestation before and after RTX therapy. Eventually, anti-DNA decreased in SLE patients after RTX injection; C3 and C4 levels did not change.
Future research should focus on several key areas to enhance the understanding and optimize using rituximab in SLE treatment. Firstly, large-scale clinical trials with rigorous study designs and diverse patient populations are essential to evaluate the efficacy and safety of rituximab compared to standard therapies. Secondly, studies exploring the optimal dosing regimens and treatment durations of rituximab in SLE are warranted to determine the most effective therapeutic approach. Additionally, investigating biomarkers that predict response to rituximab and identifying potential mechanisms of resistance or relapse after treatment cessation could provide valuable insights into personalized treatment strategies. Furthermore, long-term observational studies are needed to assess the durability of rituximab-induced remissions and its impact on disease progression and patient outcomes. Improving the management and quality of life for individuals with this difficult autoimmune disorder can be achieved by tackling these research goals and advancing the area of SLE medicines.