1. Background
Migraine is a persistent neurological disease that is determined by pulsatile and often unilateral headaches that get worse with physical activity and can be accompanied by photophobia, phonophobia, nausea, vomiting, and usually cutaneous allodynia (1). Migraine is the most recurrent neurological chief complaint in primary care. The last Global Burden Disease study showed that migraine stands second among the world's causes of disability and in first place among young women (2). Androgenetic Alopecia (AGA) is the most prevalent cause of hair loss globally, which includes 30-50% of men (male pattern hair loss or MPHL) and about 30% of women (female pattern hair loss [FPHL]) with a similar mechanism. According to AGA theory, oxidative stress leads to microinflammation in the perifollicle by accumulating pro-inflammatory cytokines. Additionally, OS and high androgen levels work together and interfere with the Corticotropin-releasing hormone function and cortisol levels (3).
Several studies have pointed to migraine headaches and female-pattern baldness as sex-conditioned inherited disorders. Considering the role of sex hormones (androgen) in female-pattern hair loss as a classic example of a sex-conditioned inherited disorder, Wang et al. proposed that the disproportionate distribution of migraine among men and women may be in a similar way affected by serum levels of sex hormones (in this case, estrogen) (4). Lemos et al. also identified gender as a contributing risk factor for migraine (5). In the same way, a review in 2022 highlighted the role of sex hormones, especially estrogen, in migraine (6).
2. Objectives
Based on previously mentioned studies and since hormonal changes are considered risk factors for migraine, this study aims to evaluate the unstudied prevalence of migraine headaches in women with AGA.
3. Methods
3.1. Data Collection
In this cross-sectional case-control study, participants included women referred to the dermatology outpatient clinics of Shiraz University of Medical Sciences in 2020. The participants were divided into two groups: (1) the case group, which clinically and by the aid of dermoscopy were diagnosed as female pattern hair loss (AGA), and (2) the control group, consisting of women of similar age without this disorder. Female-pattern hair loss was evaluated using the Ludwig classification for assessing AGA.
3.2. Clinical Monitoring
Demographic data were recorded, including age, duration of FPHL, the intensity of FPHL according to classification, and medication history. The patients were inquired about their history of migraine and the Persian versions (7). The participants completed standard questionnaires, including the Migraine Screen Questionnaire (MS_Q) and the migraine disability assessment test (MIDAS). The Visual Analog scale (VAS) was also used to determine the severity of migraine headaches. Migraine disability assessment is a reliable short questionnaire for assessing headache-related disabilities, such as migraine (the Persian version's internal consistency reliability for migraine = 0.82, validity > 0.4) (8). This questionnaire contains five questions based on a patient's pain and disability. The questionnaire divides patients into four groups: (1) minimal or no disability (score 0-5), (2) mild disability (score 5 - 10), (3) moderate disability (score 11 - 20), and (4) severe disability (score 21 and higher). On the other hand, MS_Q is another valid questionnaire which, according to a recent study, its Persian version has standard fit indices (TLI = 0.98, IFI = 0.99, CFI = 0.99, NFI = 0.98, RMSEA = 0.03) and internal consistency reliability of 70% (7). This questionnaire diagnoses migraine when four or more answers to questions are positive. After patients were determined to have migraines based on the mentioned questionnaires, they were referred to an expert neurologist for verification, but they showed reluctance to have an appointment due to the COVID-19 pandemic. Based on VAS, the patients scored pain intensity from 1 to 10. After verifying the gathered information, SPSS Version 26 was used to analyze the data.
3.3. Statistical Analysis
Based on the prevalence of migraine in women of the general population (17%) (9). and women with PCOs (44.5%) (10) and assuming first type alpha error and power to be 5% and 80%, respectively, 42 participants were required for each case and control group. The chi-squared test, t-test, Mann-Whitney test, and Spearman's rank-order correlation were used to compare case and control groups. P < 0.05 was considered statistically significant, and gathered data were analyzed using SPSS version 26.0 software.
4. Results
4.1. Demographic Data
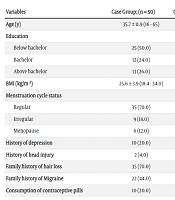
A total of 100 subjects who met the inclusion criteria were investigated. The case group consisted of 50 females with AGA with a mean age of 35.7 (SD: 11.9) years. In addition, 50 females having no evidence of AGA with a mean age of 35.4 (SD: 12.0) were considered as the control group. The mean BMI of participants in the case and control groups was 25.6 (SD: 3.9) and 25.5 (SD: 4.0), respectively. The education level of half of the patients in the case group (25 females, 50%) was below a bachelor, and most subjects in the control group had bachelor's degrees (22 females, 44%). There was no significant difference between the case and control groups concerning age (P = 0.875) and BMI (P = 0.935), but there was a significant difference concerning education (Table 1).
| Variables | Case Group; (n = 50) | Control Group; (n = 50) | P-Value |
|---|---|---|---|
| Age (y) | 35.7 ± 11.9 (16 - 65) | 35.4 ± 12.0 (17 - 64) | 0.875 |
| Education | 1.000 | ||
| Below bachelor | 25 (50.0) | 15 (30.0) | |
| Bachelor | 12 (24.0) | 22 (44.0) | |
| Above bachelor | 13 (26.0) | 13 (26.0) | |
| BMI (kg/m2) | 25.6 ± 3.9 (18.4 - 34.0) | 25.5 ± 4.0 (18.8 - 38.1) | 0.935 |
| Menstruation cycle status | 0.559 | ||
| Regular | 35 (70.0) | 40 (80.0) | |
| Irregular | 9 (18.0) | 4 (8.0) | |
| Menopause | 6 (12.0) | 6 (12.0) | |
| History of depression | 10 (20.0) | 8 (16.0) | 0.603 |
| History of head injury | 2 (4.0) | 4 (8.0) | 0.678 |
| Family history of hair loss | 35 (70.0) | 2 (4.0) | < 0.001 |
| Family history of Migraine | 22 (44.0) | 17 (34.0) | 0.305 |
| Consumption of contraceptive pills | 10 (20.0) | 8 (16.0) | 0.603 |
a Values are expressed as mean ± SD (min - max) or No. (%).
Women in the case and control groups mainly had regular menstruation cycles: 70% (35 females) in the case group and 80% (40 females) in the control group. Ten subjects (20%) in the case group and 8 (16%) subjects in the control group had a previous history of depression. Two (4%) subjects in the case group and four (8%) subjects in the control group reported a history of head injury. A total of 22 females (44%) in the case group and 17 females (34%) in the control group had a family history of migraines. 20% of cases (10 females) and 16% of controls (8 females) were consuming contraceptive pills. There was no significant difference between case and control groups in menstruation cycle status (P = 0.324), history of depression (P = 0.603), history of head injury (P = 0.678), family history of migraine (P = 0.305), and consumption of contraceptive pills (P = 0.603). Positive family history of hair loss in case group 70% (35 females) was significantly more than the control group 4% (two females) (P < 0.001).
The age distribution at onset of hair loss ranged from 13 to 60 years in the case group, and the mean age at onset of hair loss was 26.3 (SD: 12.3) years. There was no significant correlation between age at onset of hair loss and BMI in the case group (Pearson correlation coefficient = 0.141, P = 0.330). The intensity of hair loss according to the Ludwig classification scale in the case group was calculated. As a result, most patients (52%) in the case group were classified as Ludwig one score, 28% as score 2, and 20% as score 3.
4.2. Comparing the Patients in the Case and Control Group
As shown in Table 2, 40% of females (n = 20) in the case group and 38% (n = 19) in the control group reported headaches. The difference was not significant between the case and control groups (P = 0.838). In addition, six females (12%) in the case group and 11 females (22%) in the control group believed that their headache was related to premenstrual syndrome. There was no significant difference in the rate of headache due to premenstrual syndrome between the two groups (P = 0.183). Regarding migraine signs, four females (8%) in the case group and four females (8%) in the control group experienced visual signs, three females (6%) in the case group and four females (8%) in the control group experienced sensory signs, and none of the participants experienced verbal signs. No significant difference was found in triple signs of migraine between case and control groups (P > 0.05) Table 2.
| Variables | Case Group; (n = 50) | Control Group; (n = 50) | P-Value |
|---|---|---|---|
| Headache | 20 (40.0) | 19 (38.0) | 0.838 |
| Headache due to premenstrual syndrome | 6 (12.0) | 11 (22.0) | 0.183 |
| Migraine sign | |||
| Visual | 4 (8.0) | 4 (8.0) | 1.000 |
| Sensory | 3 (6.0) | 4 (8.0) | > 0.999 |
| Verbal | 0 (0.0) | 0 (0.0) | - |
a Values are expressed as No. (%).
According to the MS_Q Questionnaire, seven females (14%) in the case group and six females (12%) in the control group had migraines. There was no significant difference between the case and control groups in the prevalence of migraine (P = 0.766) (Table 3).
| Variables | No Migraine | Migraine | P-Value |
|---|---|---|---|
| Case Group | 43 (86.0) | 7 (14.0) | 0.766 |
| Control Group | 44 (88.0) | 6 (12.0) |
a Values are expressed as No. (%).
4.3. Comparing the Patients with Migraine in Both Case and Control Groups
Characteristics of study participants with migraines were summarized in Table 4. The mean age of the case group was 30.0 (SD: 8.0) years, and the mean age of the control group was 33.2 (SD: 10.8). The mean BMI of participants in the case and control groups was 28.4 (SD: 4.5) and 26.5 (SD: 6.3), respectively. Three females (42.9%) in the case group had bachelor's degrees, and three females (42.9%) had below bachelor's degrees. The education level of half of the patients in the control group (3 females, 50%) was below bachelor. There was no significant difference in patients with migraine between the case and control groups concerning age (P = 0.668), BMI (P = 0.391), and education (P = 1.000). Women in the case and control groups mainly had regular menstruation cycles: 85.7% (six females) in the case group and 66.7% (four females) in the control group. One subject (14.3%) in the case group and four (66.7%) subjects in the control group had a previous history of depression. Three females (42.9%) in the case group and three females (50%) in the control group had a family history of migraines. There was no significant difference in patients with migraine between case and control groups in menstruation cycle status (P = 0.559), history of depression (P = 0.103), and family history of migraine (P = 1.000). Positive family history of hair loss in the case group with migraine 71.4% (five females) was significantly more than the control group with migraine 0% (P = 0.021). Consumption of contraceptive pills in the control group with migraine 66.7% (four females) was significantly more than the case group with migraine 0% (P = 0.021). No one in the case and control groups with migraines had a history of head injury (Table 4).
| Variables | Case Group; (n = 7) | Control Group; (n = 6) | P-Value |
|---|---|---|---|
| Age (y) | 30.0 ± 8.0 (19 - 40) | 33.2 ± 10.8 (18 - 45) | 0.668 |
| Education | 1.000 | ||
| Below bachelor | 3 (42.9) | 3 (50.0) | |
| Bachelor | 3 (42.9) | 2 (33.3) | |
| Above bachelor | 1 (14.3) | 1 (16.7) | |
| BMI (kg/m2) | 28.4 ± 4.5 (22.4 - 34.0) | 26.5 ± 6.3 (20.2 - 38.1) | 0.391 |
| Menstruation cycle status | 0.559 | ||
| Regular | 6 (85.7) | 4 (66.7) | |
| Irregular | 1 (14.3) | 2 (33.3) | |
| History of depression | 1 (14.3) | 4 (66.7) | 0.103 |
| History of head injury | 0 (0.0) | 0 (0.0) | - |
| Family history of hair loss | 5 (71.4) | 0 (0.0) | 0.021 |
| Family history of Migraine | 3 (42.9) | 3 (50) | 1.000 |
| Consumption of contraceptive pills | 0 (0.0) | 4 (66.7) | 0.021 |
a Values are expressed as mean ± SD (min-max) or No. (%).
Table 5 describes headaches in participants with migraines. No females in the case group and five females (83.3%) in the control group believed that their headache was related to their menstrual period. There was a significant difference in the rate of headache due to menstrually-related migraine between the two groups (P = 0.005). Regarding migraine signs, three females (42.9%) in the case group and two females (33.3%) in the control group experienced visual signs, two females (28.6%) in the case group and two females (33.3%) in the control group experienced sensory signs. None of the participants experienced verbal signs. No significant difference was found in triple signs of migraine between case and control groups (P > 0.05) (Table 5).
| Variables | Case Group; (n = 7) | Control Group; (n = 6) | P-Value |
|---|---|---|---|
| Menstrually-related migraine | 0 (0.0) | 5 (83.3) | 0.005 b |
| Migraine sign | |||
| Visual | 3 (42.9) | 2 (33.3) | 1.000 |
| Sensory | 2 (28.6) | 2 (33.3) | 1.000 |
| Verbal | 0 (0.0) | 0 (0.0) | - |
a Values are expressed as No. (%).
b P < 0.05 was considered statistically significant.
The intensity of migraine was evaluated based on the MIDAS questionnaire and VAS. The majority of participants had severe disability, 57.1% (four females) in the case group and 66.7% (four females) in the control group. There was no significant difference in migraine disability between the case and control groups (P = 0.559). In addition, there was no significant difference in VAS between case (6.0 ± 3.3) and control (5.0 ± 2.3) groups (P = 0.270).
There was no significant correlation between Ludwig score and migraine disability score in the case group with migraine (Spearman correlation coefficient = 0.367, P = 0.419). However, there was a significant positive correlation between Ludwig score and VAS in the case group with migraine (Spearman correlation coefficient = 0.844, P = 0.017). Patients with higher Ludwig scores had higher VAS (data not shown).
4.4. Comparing the Patients with Androgenetic Hair Loss with and Without Migraine Headache
Mean age of onset, BMI, Ludwig score, and mean Ludwig score were compared between patients with AGA and migraine and patients with AGA without migraine. The results showed no significant difference in mean age of onset (P = 0.743), mean BMI (P = 0.069), Ludwig score (P = 0.662), and mean Ludwig score (P = 1.000) in the case group.
5. Discussion
The limitation of this study was the small population that was included and the lack of measurement of Androgen levels. Thus, further studies should be conducted with larger populations and androgen level measurements to get more accurate results and judge precisely.
The relationship between migraine and female pattern hair loss is unclear. Still, migraine affects women much more commonly than men, and a relationship between these two disorders is to be suspected, considering the role that sex hormones play in the occurrence of both male and female AGA.
Both migraine and AGA have different incidence rates in different stages of a person’s life. While in the present study, the onset of hair loss appeared to range from 13 to 60 years (14 to 38 years in patients with migraine), migraine gradually increases with age, reaching its peak during the fourth decade of an individual’s life before starting to decline (11). FAGA, on the other hand, continues to increase with age, starting from 3 - 12% after puberty and before the age of 40, going up to 29 - 56% in women above 70 (12). Several studies have mentioned sex hormones playing a role in the incidence of migraine. Particularly, estrogen has been found to affect the pathogenesis of this headache disorder greatly. For instance, discoveries show that estrogen withdrawal is the leading cause of menstrual migraine, and estrogen level variations have a role in migraine pathophysiology (12). In addition, in a recent study, about 60% of women reported their migraines to be associated with menses (13). However, in another study consisting of 85 females with MM, despite 35.3% of them reporting headaches by the end of menstruation, it was suggested that this type of headache is most probably related to transient anemia caused by blood loss instead of hormonal changes (14). On the other hand, migraine with aura is believed to be much more related to high estrogen levels, unlike MM (15). While the mechanisms by which sex hormones affect migraine are beyond the scope of this paper, findings indeed suggest both male and female hormones have an important effect on the incidence of migraine and other primary headaches (15). The etiopathogenesis of FPHL is quite complex, with genetic, hormonal, and environmental factors playing a key role. The role of androgens in the development of FPHL is not yet clear. Female pattern hair loss occurs in females with normal levels of circulating androgens, and a genetic predisposition may be involved in women without elevated androgen levels. This genetic disposition permits normal circulating androgen levels to act on follicular target cells, especially sensitized by binding to specific intracellular androgen receptors (16). The case group had a 70% positive family history of AGA, while the control group had 4%. FPHL may also be caused by hyperandrogenism, polycystic ovaries (PCO), and, to a lesser extent, increased androgen/tarragon ratios in women (17). Although some female hormones have been mentioned in the etiology of migraine, androgens have gained very little attention. Studying migraine and serum levels of androgens, Mattson P. found no evidence linking to a connection between different serum levels of androgens and the occurrence of migraine (18). In our study, no significant correlation was observed between migraine and AGA. Furthermore, a cross-sectional study focusing on allopregnanolone, progesterone, and testosterone reported that serum levels of progesterone and testosterone stayed the same in MM and postmenopausal migraine. In contrast, low allopregnanolone levels seemed to be significantly correlated (19). A common feature of PCO is AGA, though it is not as common as hirsutism17. A study in the USA reported the prevalence of PCO to be 22% in women with AGA (20). Regarding hirsutism, Birch et al. found that women with this disorder, either with or without FPHL, had a significantly increased BMI, which was shown to be irrelevant to FPHL (21). In addition, BMI was not found to maintain any significant correlation with age at onset of hair loss in any participants. In contrast, the number of participants in our study was partially based on the prevalence of PCO. A similar earlier study also concluded that migraine and PCO are not closely related (10). The few significant results we encountered were that in patients with FPHL and migraine headaches, the higher the Ludwig score, the more the patient suffered headaches with higher VAS scores. Moreover, menstrual-related migraine headaches were significantly more prevalent in the control group than in patients with FPHL (P = 0.005). This can partially be explained by either the frequent use of finasteride in FPHL patients or the supposed stronger presence of anovulatory cycles in these patients. Delaruelle et al. showed that almost half of the female migraine patients report an association between headache and their menstrual cycle based on the “Estrogen withdrawal hypothesis.” Depending on whether migraine occurs exclusively during the perimenstrual period or also at other times, the International Headache Society (IHS) distinguishes a pure menstrual migraine from a menstrual-related migraine (16). Although the onset of hair loss appeared to happen at a lower median age in women with migraine (23.4 vs. 26.3), the present study did not face significant differences in the prevalence of migraine in women with AGA compared with those without this disorder. A limitation of this study includes the number of available participants and the lack of serum hormonal assay in patients and control group. Moreover, some patients might not have properly filled out the questionnaires. While a decent understanding of the relationship between migraine and AGA, along with sex hormones, has not been properly established, it is advised that future studies with larger databases, including complete detailed sex hormonal assays, be conducted to help shed light on this matter.
5.1. Conclusions
No significant evidence suggested a correlation between migraine and female-pattern hair loss. Therefore, future studies with more extensive databases, including complete detailed sex hormonal assays, should be conducted to help shed light on this matter.