1. Context
Non-alcoholic fatty liver disease (NAFLD) is one of the leading causes of chronic liver disorders (1, 2). It is estimated that 25% of the global population suffers from NAFLD (3), which is characterized by the accumulation of triglycerides in liver hepatocytes (4). Conditions such as obesity, metabolic syndrome, and type 2 diabetes can contribute to its development (5). The consequences of NAFLD range from non-alcoholic steatohepatitis (NASH) to fibrosis, cirrhosis, hepatocellular carcinoma, and eventually death (3, 6). A recent meta-analysis reported that patients with NAFLD have an increased risk of cardiovascular disease, stroke, breast cancer, and colorectal cancer (7).
Diet, in addition to contributing to the development of NAFLD, can also play a role in its treatment. Therefore, weight loss or maintaining a healthy weight can reduce inflammation and improve fatty liver (8, 9). Healthy dietary patterns, which include plenty of fruits, vegetables, and whole grains while limiting saturated fatty acids, trans fats, and simple sugars, have been suggested to help prevent and manage chronic diseases (10). Studies have shown that an increased intake of energy-dense foods, such as those high in saturated fats and simple sugars, is associated with the development of fatty liver disease (9). Evidence from observational studies suggests that a Western diet is linked to the occurrence of NAFLD, while adherence to a healthy dietary pattern is associated with a reduced risk of developing the disease (11).
Meta-analyses and systematic reviews have examined the effects of dietary patterns such as low-carbohydrate diets, Mediterranean diets, and calorie-restricted diets on the progression of NAFLD. Given the increasing prevalence of NAFLD and research in this area, an umbrella review of systematic reviews and meta-analyses is warranted to determine the most effective dietary approach for improving, preventing, and reducing the incidence of NAFLD.
2. Evidence Acquisition
This umbrella review was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (12).
2.1. Protocol and Registration
The protocol for this umbrella review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022377459).
2.2. Search Strategy, Inclusion, and Exclusion Criteria
The research question and the inclusion and exclusion criteria were developed using the Population, Interventions, Comparators, Outcomes, and Study Design (PICOS) framework (Appendix 1 in Supplementary File). Two independent researchers conducted a systematic search of electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar, up to March 3, 2023. Both Medical Subject Headings (MeSH) and non-MeSH keywords were used (Appendix 2 in Supplementary File), and references from relevant articles were reviewed to avoid missing any pertinent studies. No restrictions were imposed based on language, time, or location in the systematic search.
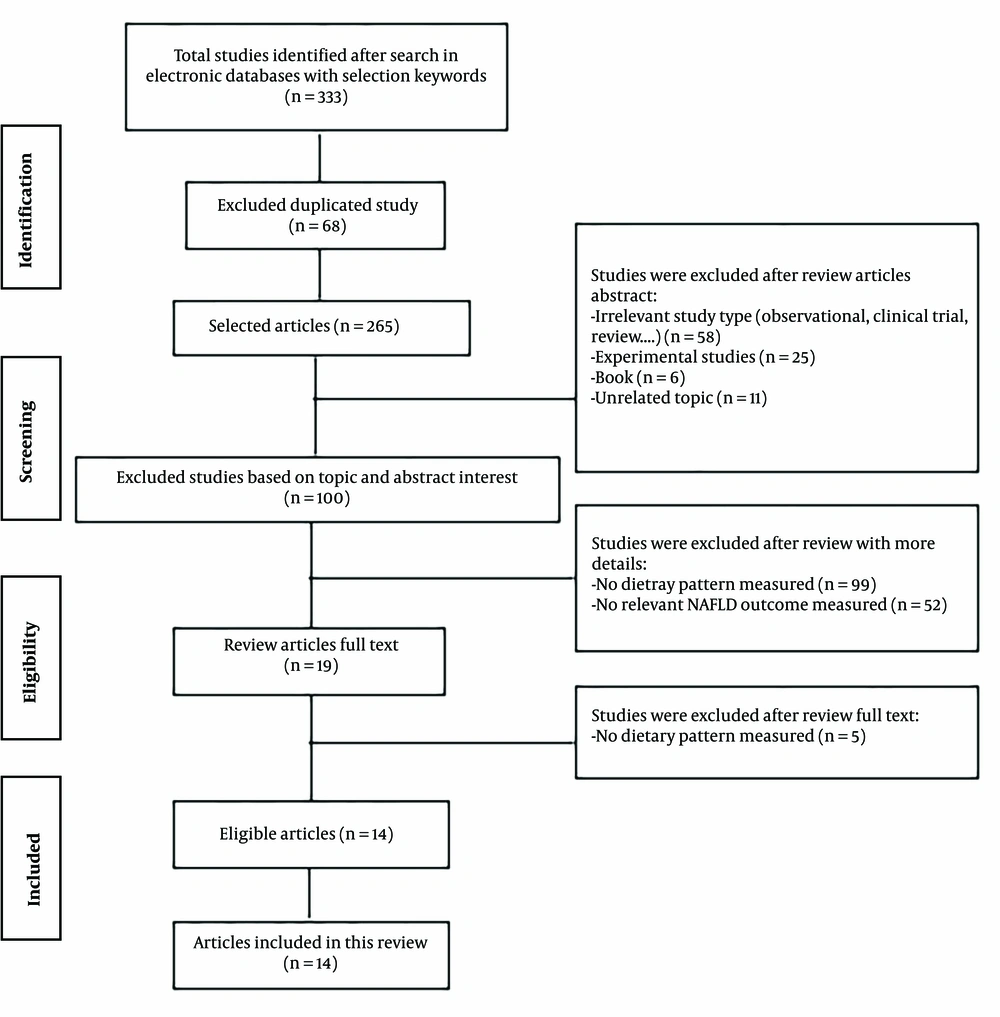
Eligible studies for inclusion were systematic reviews and meta-analyses that met the following criteria: (1) calculated random-effects sizes with a 95% confidence interval (CI); (2) conducted on adults; and (3) included dietary patterns as the exposure. Non-alcoholic fatty liver disease was considered the outcome of interest. The initial search yielded 333 articles, of which 68 were duplicates. After removing these duplicates, 265 articles remained. Of these, 58 were excluded because they were irrelevant study types (observational, clinical trials, reviews, etc.), 25 were experimental studies, six were books, and 11 were unrelated. A total of 165 articles were assessed in more detail, and 151 additional studies were excluded for the following reasons: Ninety-nine did not examine dietary patterns as the exposure, and 52 did not consider NAFLD as an outcome. After further screening, 19 studies remained, but five were excluded because they did not examine dietary patterns. Ultimately, 14 studies were eligible for inclusion in this review (Figure 1).
2.3. Data Extraction
Data from the eligible studies were independently extracted by two researchers using a standardized data collection checklist. This checklist, formatted as an Excel file, included the following information: Author names, year of publication, country of study, number of included studies, type of studies, sample size, age of participants, type of diet, random-effects size (CI 95%), I² statistic, and Egger test P-values. Additionally, data related to NAFLD outcomes, including hepatic steatosis, intrahepatic fat (IHF), Fatty Liver Index (FLI), and related biochemical measures [alanine transaminase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT)], were extracted. Any disagreements were resolved through discussion after completing the forms.
2.4. Quality Assessment
Two researchers independently evaluated the quality of the included studies. In cases of disagreement, a third researcher re-evaluated the studies. The quality assessment was conducted using a measurement tool to assess systematic reviews 2 (AMSTAR-2). A measurement tool to assess systematic reviews 2 assesses the quality of studies by asking the following 16 questions: (1) PICOS components; (2) review protocol; (3) study designs; (4) comprehensive literature search strategy; (5) study selection in duplicate; (6) data extraction in duplicate; (7) list of excluded studies and justification for exclusions; (8) study characteristics; (9) satisfactory techniques for assessing risk of bias; (10) source of funding; (11) appropriate statistical methods; (12) assessing the potential impact of bias on results; (13) accounting for bias when interpreting and discussing results; (14) a satisfactory explanation for and discussion of any heterogeneity; (15) publication bias and related discussion; and (16) conflict of interest.
Each item was rated as "Yes" when fully addressed, "Partial Yes" when it was only partially described, "No" when it was not done, and "Unclear" when it was uncertain if it had been completed (Appendix 3 in Supplementary File).
3. Data Synthesis
The pooled effect sizes from meta-analyses of randomized controlled trials (RCTs) for each outcome before and after dietary interventions were converted into standardized mean differences (SMDs) with 95% CI (Tables 1 and 2). Forest plots were created for each outcome based on the dietary pattern type and their effects on NAFLD-related outcomes. All statistical analyses were performed using Stata software version 11.2 (StataCorp, College Station, TX).
| Author, Year, Refrence | Country | Total Number of Study | RCT | Sample Size | Age (y) | Kind of Diet | SMD (CI 95%) | I², (%) | Egger Test P-Value |
|---|---|---|---|---|---|---|---|---|---|
| Hepatic steatosis | |||||||||
| Akhlaghi et al., 2020, (13) | Iran | 13 | 4 | 272 | - | Mediterranean diet | -1.43 (-3.4, 0.58) | 68.9 | No publication bias |
| Garcez et al., 2021, (14) | Brazil | 6 | 2 | 127 | 18 - 81 | low-carbohydrate diet | 0.39 (-1.6, 2.37) | 87.56 | NR |
| Haigh et al., 2022, (15) | UK | 26 | 4 | 233 | 48.3 ± 5.9 | Mediterranean diet | -0.4 (-0.66, 1.61) | 76 | No publication bias |
| 4 | 257 | 48.3 ± 5.9 | calorie-restricted | -5.65 (-7.46, -3.54) | 0 | No publication bias | |||
| FLI | |||||||||
| Haigh et al., 2022, (15) | UK | 26 | 4 | 228 | 48.3 ± 5.9 | Mediterranean diet | -4.86 (-6.86, -2.86) | 71 | No publication bias |
| 1 | 260 | 48.3 ± 5.9 | Calorie-restricted | -2.54 (-4.54, -0.54) | NR | No publication bias | |||
| Kawaguchi et al., 2021, (16) | Japan | 6 | 3 | 140 | 33 - 56 | Mediterranean diet | -1.06 (-1.95, -0.17) | 76 | Significant bias for FLI |
| IHF | |||||||||
| Ahn et al., 2018, (17) | South Korea | 11 | 4 | 22 | NR | Low-carbohydrate diet | 1.7 (-0.3,3.68) | 91 | NR |
| Bueno et al., 2019, (18) | Brazil | 4 | 4 | 183 | > 18 | Low-carbohydrate diet | -2.64 (-4.64, -0.64) | 68.30 | NR |
| Henschel, 2021, (19) | USA | 11 | 4 | 22 | NR | Low-carbohydrate diet | 2.38 (0.38,4.36) | NR | NR |
| Haghighatdoost et al., 2016, (20) | Iran | 10 | 4 | 50 | > 18 | Low-carbohydrate diet | -3.51 (-5.5, -1.51) | 83.20 | 0.34 |
| Houttu et al., 2021, (21) | Finland | 8 | 2 | 72 | 38 - 55 | Mediterranean diet | -0.57 (-1.04, -0.1) | 0 | Did not conducted |
| Liver stiffness | |||||||||
| Haigh et al., 2022, (15) | UK | 26 | 5 | 271 | 48.3 ± 5.9 | Mediterranean diet | -1.99 (-3.97, 0) | 87 | No publication bias |
| 2 | 193 | 48.3 ± 5.9 | Calorie-restricted | -2.64 (-4.6, -0.67) | 0 | No publication bias | |||
| Yin et al., 2021, (22) | China | 6 | 2 | 343 | ≥ 18 | Intermittent fasting | 0.12 (-1.85,2.1) | 0 | Visual inspection of funnel plots |
| Kawaguchi et al., 2021, (16) | Japan | 6 | 2 | 72 | 33 - 56 | Mediterranean diet | -0.67 (-1.7,0.36) | 75 | No publication bias |
Abbreviations: NR, not reported; FLI, Fatty Liver Index; RCT, randomized controlled trials; SMD, standard mean difference; IHF, intrahepatic fat.
| Author, Year, Refrence | Country | Total Number of Study | RCT | Sample Size | Age (y) | Kind of Diet | SMD (CI 95%) | I², (%) | Egger Test P-Value |
|---|---|---|---|---|---|---|---|---|---|
| ALT | |||||||||
| Ahn et al., 2018, (17) | South Korea | 11 | 5 | 33 | NR | Low-carbohydrate diet | 1.57 (-0.4, 3.56) | 73 | NR |
| Henschel et al., 2021, (19) | USA | 11 | 5 | 33 | NR | Low-carbohydrate diet | 1.69 (-0.3, 3.69) | NR | NR |
| Katsagoni et al., 2017, (23) | Greece | 20 | 3 | > 18 | Moderate-carbohydrate diet | 0.05 (-0.27, 0.36) | 0 | No publication bias | |
| Garcez et al., 2021, (6) | Brazil | 6 | 4 | 204 | 18 - 81 | Low-carbohydrate diet | −0.02 (-2.01, 1.96) | 0 | NR |
| Haghighatdoost et al., 2016, (20) | Iran | 10 | 11 | 230 | > 18 | Low-carbohydrate diet | -1.02 (-3.01,0.98) | 87.90 | 0.52 |
| Houttu et al., 2021, (21) | Finland | 8 | 2 | 113 | 38 - 55 | Hypo-caloric diet | -1.09 (-1.49, 0.69) | 0 | Did not conducted |
| 2 | 72 | 38 - 55 | Mediterranean diet | 0.07 (-0.39, 0.53) | 0 | Did not conducted | |||
| Haigh et al., 2022, (15) | UK | 26 | 9 | 413 | 48.3 ± 5.9 | Mediterranean diet | -2.38 (-4.38, -0.38) | 81 | No publication bias |
| 8 | 667 | 48.3 ± 5.9 | Calorie-restricted | -4.24 (-6.25, -2.25) | 0 | No publication bias | |||
| Sangouni et al., 2021, (24) | Iran | 10 | 8 | 498 | ≥ 18 | Mediterranean diet | -1.61 (-3.57,0.39) | NR | 0.95 |
| Yin et al., 2021, (22) | China | 6 | 2 | 194 | ≥ 18 | Intermittent fasting | -6.08 (-8.09, -4.09) | 0 | Visual inspection of funnel plots |
| Kawaguchi et al., 2021, (16) | Japan | 6 | 6 | 295 | 33-56 | Mediterranean diet | -0.79 (-2.21,0.62) | 96 | No publication bias |
| AST | |||||||||
| Ahn et al., 2018, (17) | South Korea | 11 | 4 | 54 | NR | Low-carbohydrate diet | 1.47 (-0.43, 3.52) | 77 | NR |
| Henschel et al., 2021, (19) | USA | 11 | 4 | 54 | NR | Low-carbohydrate diet | 1.65 (-0.35, 3.65) | NR | NR |
| Katsagoni et al, 2017, (23) | Greece | 20 | 4 | > 18 | Moderate-carbohydrate diet | 0.28 (-0.26, 0.82) | 55.60 | No publication bias | |
| Garcez et al., 2021, (14) | Brazil | 6 | 5 | 130 | 18 - 81 | Low-carbohydrate diet | −0.81 (-2.81, 1.19) | 0 | NR |
| Haghighatdoost et al., 2016, (20) | Iran | 10 | 10 | 216 | > 18 | Low-carbohydrate diet | -0.81 (-2.81, 1.19) | 61.40 | 0.38 |
| Houttu et al., 2021, (21) | Finland | 8 | 2 | 113 | 38 - 55 | Hypo-caloric diet | -0.75 (-1.23, 0.23) | 45 | Did not conducted |
| Haigh et al., 2022, (15) | UK | 26 | 6 | 314 | 48.3 ± 5.9 | Mediterranean diet | -1.8 (-3.8, 0.2) | 81 | No publication bias |
| 7 | 628 | 48.3 ± 5.9 | Calorie-restricted | -1.46 (-3.44, 0.54) | 53 | No publication bias | |||
| Sangouni et al., 2021, (24) | Iran | 10 | 4 | 197 | ≥ 18 | Mediterranean diet | -2.2 (-4.09, -0.2) | NR | 0.3 |
| Yin et al., 2021, (22) | China | 6 | 2 | 194 | ≥ 18 | Intermittent fasting | -7.57 (-9.53, -5.55) | 0 | Visual inspection of funnel plots |
| GGT | |||||||||
| Haghighatdoost et al., 2016, (20) | Iran | 10 | 6 | 92 | >18 | low-carbohydrate diet | -0.72 (-2.72,1.28) | 99.40 | 0.86 |
| Houttu et al., 2021, (21) | Finland | 8 | 3 | 154 | 38-55 | Mediterranean diet | 0.04 (-0.28,0.35) | 0 | Did not conducted |
| Sangouni et al., 2021, (24) | Iran | 10 | 9 | 513 | ≥ 18 | Mediterranean diet | -2.08 (-4.08, -0.08) | NR | 0.44 |
Abbreviations: NR, not reported; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; RCT, randomized controlled trials; SMD, standard mean difference.
4. Results
4.1. Characteristics of Included Systematic Reviews and Meta-analyses
All 14 included studies were conducted between 2016 and 2023, with four conducted in Iran (13, 20, 24, 25), two in Brazil (14, 18), and the others in Cleveland (26), South Korea (17), the UK (15), the USA (19), Finland (21), Japan (16), China (22), and Greece (23). Eleven studies performed meta-analyses exclusively on RCTs (14-24), while two only analyzed observational studies (case-control, cross-sectional, and cohort) (25, 26). Akhlaghi et al. (13) meta-analyzed both observational and interventional studies. Meta-analyses of RCTs had sample sizes ranging from 22 to 667, while observational studies included 301 to 16,823 participants. Bueno et al.'s (18) and Henschel et al.'s (19) studies were letters addressing the study by Ahn et al. (17), who had analyzed the data differently. Consequently, Ahn et al.’s study was excluded in favor of the findings from the other studies (17).
Three of the included studies assessed hepatic steatosis (13-15), two assessed the FLI (15, 16), and five evaluated IHF (17-21) as primary outcomes. Regarding biochemical factors related to liver function, ten studies evaluated ALT (14-17, 19-24), nine assessed AST (14, 15, 17, 19-24), and three measured GGT (20, 21, 24). The examined dietary patterns included the Mediterranean diet, low-carbohydrate diet, moderate-carbohydrate diet, calorie-restricted, hypocaloric diet, intermittent fasting, gluten-free diet, prudent diet, and Western diet (Tables 1 - 3).
| Author, Year, Refrence | Country | Observational Study (N) | Sample Size | Age (y) | Kind of diet | OR (CI 95%) | P Random | I² | Egger Test P-Value |
|---|---|---|---|---|---|---|---|---|---|
| Aggarwal et al., 2021, (26) | Cleveland | 5 | 301 | > 15 | Gluten-free diet | 0.214 (0.096, 0.413) | < 0.01 | 97% | NR |
| Akhlaghi et al., 2019, (13) | Iran | 7 | 16823 | NR | Mediterranean diet | 0.95 (0.9 - 1) | 0.05 | 68.1% | 0.002 |
| Hassani Zadeh et al., 2020, (25) | Iran | 5 | 3057 | NR | Mediterranean diet | 0.772 (0.602, 0.989) | 0.041 | NR | 0.04 |
| 14 | 13023 | NR | Prudent diet | 0.786 (0.719, 0.859) | < 0.001 | 12.41% | 0.15 | ||
| 10 | 8787 | NR | Western dietary | 1.567 (1.277, 1.922) | < 0.001 | 46.96% | 0.01 |
Abbreviation: NR, not reported.
4.2. Hepatic Steatosis
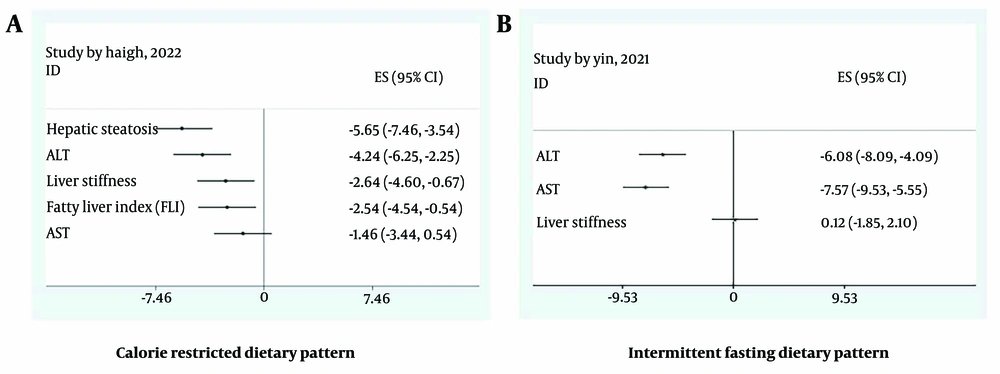
Three meta-analyses (13-15) investigated the effects of the Mediterranean, calorie-restricted, and low-carbohydrate diets on hepatic steatosis (Table 1). As shown in the forest plot (Appendix 4A in Supplementary File), the most significant effect on hepatic steatosis was related to the calorie-restricted diet (SMD: -5.65; CI 95%: -7.46, -3.54), with an I² of 0.
4.3. Fatty Liver Index
This index was evaluated in meta-analyses by Haigh et al. (15) and Kawaguchi et al. (16), both of which showed that the Mediterranean diet significantly reduced the FLI (Table 1 and Appendix 4B in Supplementary File).
4.4. Intrahepatic Fat
The findings indicated that the low-carbohydrate diet improved IHF more effectively than the Mediterranean diet (Appendix 4C in Supplementary File). Ahn et al. initially reviewed the low-carbohydrate diet and did not observe a significant effect (17). However, in 2019, Bueno et al. (18) published a letter modifying Ahn’s analysis, which revealed a significant effect. Henschel et al. supported these findings in another letter, and Bueno’s study was ultimately included in the analyses (19) (Table 1).
4.5. Liver Stiffness
Among the studied diets, the calorie-restricted diet had the most significant effect on improving liver stiffness (SMD: -2.64; CI 95%: -4.6, -0.67), with an I² of 0 (15) (Table 1 and Appendix 4D in Supplementary File).
4.6. Biochemical Indicators Related to Liver Function
Ten studies assessed the effects of dietary patterns (low-carbohydrate, moderate-carbohydrate, hypocaloric, calorie-restricted, intermittent fasting, and Mediterranean diet) on serum ALT levels in patients with NAFLD (Table 2). The forest plot (Appendix 5 in Supplementary File) illustrates the effects of these dietary patterns on ALT. Both calorie-restricted (SMD: -4.24; CI 95%: -6.25, -2.25) and intermittent fasting (SMD: 6.08; CI 95%: -8.09, -4.09) diets showed the most significant improvements in ALT levels.
Nine studies evaluated serum AST levels in NAFLD patients. The forest plot (Appendix 6 in Supplementary File) showed that the intermittent fasting diet (SMD: -7.57; CI 95%: -9.53, -5.55) significantly improved AST levels.
Only three studies, including Sangouni et al. (24), assessed serum GGT levels in NAFLD patients. In 2021, a meta-analysis of nine RCTs demonstrated that the Mediterranean diet significantly improved GGT levels (SMD: -2.08; CI 95%: -4.08, -0.08) (Table 2 Appendix 7 in Supplementary File).
4.7. Calorie-Restricted Diet
The effects of a calorie-restricted diet were evaluated across several liver parameters, including hepatic steatosis, FLI, liver stiffness, ALT, and AST (Figure 2A). As shown in Forest Plot 2, the most significant effect was on hepatic steatosis (SMD: -5.65; CI 95%: -7.46, -3.54).
4.8. Intermittent Fasting Diet
In a meta-analysis by Yin et al. (22), intermittent fasting (including alternate-day fasting and other forms of periodic caloric restriction) was assessed for its effects on ALT, AST, and liver stiffness. Figure 2B shows that this dietary pattern significantly improved liver enzymes but had no significant effect on liver stiffness.
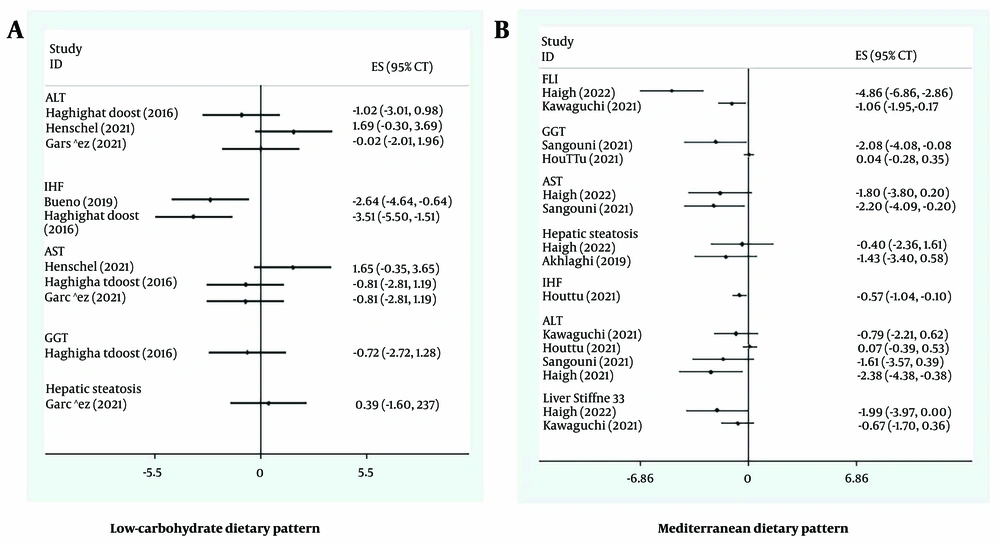
4.9. Low-carbohydrate Diet
Five meta-analyses investigated the effects of a low-carbohydrate diet on NAFLD-related outcomes. This diet typically reduced carbohydrate intake to 40-50% of daily energy. As shown in Figure 3A, the low-carbohydrate diet was significantly associated with reducing IHF. Studies by Bueno et al. (18) and Haghighatdoost et al. (20) supported these findings. In 2016, Haghighatdoost et al. (20) found that a low-carbohydrate diet (with less than 50% of daily energy from carbohydrates) improved IHF (SMD: -3.51; CI 95%: -5.5, -1.51). A meta-analysis by Bueno et al. (18) showed that a low-carbohydrate diet (40% of daily energy from carbohydrates) significantly reduced IHF (SMD: -2.64; CI 95%: -4.64, -0.64).
4.10. Mediterranean Diet
As depicted in Figure 3B, the Mediterranean diet significantly improved all investigated outcomes, except hepatic steatosis, where the effect was not statistically significant. However, the Mediterranean diet still showed a trend toward reducing hepatic steatosis.
5. Discussion
The findings provided an overview of the best dietary approaches to prevent NAFLD. Among the evaluated dietary patterns, the calorie-restricted diet emerged as the most effective, significantly improving all NAFLD-related outcomes. Similarly, adherence to the Mediterranean diet showed beneficial effects in improving these outcomes. Non-alcoholic fatty liver disease is currently the most common cause of chronic liver disease, and its prevalence is rising (27). At present, there are no successful pharmaceutical treatments for this condition, and several dietary interventions have been suggested (28).
The calorie-restricted diet was shown to improve hepatic steatosis, the FLI, liver stiffness, and liver enzyme levels. This dietary pattern was evaluated in a meta-analysis by Haigh et al. (15) on nine RCTs, which recommended its inclusion in the treatment strategy for NAFLD patients. Historical evidence suggests that reduced dietary intake has been associated with increased life expectancy and reduced non-communicable diseases since the early 19th century. As a result, "calorie restriction" was formally introduced in the late 1930s (29). One mechanism proposed in Asghari et al.'s study (30) suggests that a calorie-restricted diet improves sensitivity to fibroblast growth factor 21 (FGF-21), which is resistant in NAFLD patients. Caloric restriction helps regulate energy homeostasis, fat and glucose metabolism, and improves inflammatory conditions and oxidative stress-induced damage. Additionally, the beneficial effects of caloric restriction are linked to the induction of the sirtuin family, particularly SIRT1, which protects NAD+-dependent enzymes and increases organism lifespan (31). SIRT4 also plays a critical role in fatty acid oxidation in liver cell mitochondria through SIRT1 (32, 33). Moreover, caloric restriction has been shown to reduce proteins associated with liver inflammation in animal models and has beneficial effects on insulin resistance, liver steatosis, lipogenesis enzymes, mitochondrial biogenesis, collagen deposition, and endoplasmic reticulum stress (33). Therefore, the findings of this study support the use of calorie restriction in conjunction with other treatment approaches for NAFLD patients.
The present umbrella review also highlighted the Mediterranean diet's role in improving NAFLD-related outcomes. The Mediterranean diet is characterized by high consumption of plant-based foods, including fruits, vegetables, legumes, nuts, and whole grains. Olive oil is the primary source of dietary fat, and moderate consumption of red wine, fresh fish, dairy products, poultry, and eggs is recommended, while red and processed meats are limited (34). Nutritionally, this diet is rich in monounsaturated and polyunsaturated fatty acids (PUFAs) and fiber, while refined grains and foods with added sugars are limited (35). These components reduce inflammation and oxidative stress, offering preventive effects against NAFLD. The Mediterranean diet also includes PUFAs, particularly omega-3 fatty acids, and a lower ratio of omega-6 to omega-3 fatty acids, which help prevent liver fat accumulation and reduce hepatic steatosis (36, 37).
An essential component of the Mediterranean diet is olive oil, which has demonstrated anti-inflammatory and antioxidant properties in improving NAFLD (35, 38). Another crucial aspect is the high fiber content from whole grains and complex carbohydrates, which have a Low Glycemic Index and are low in fructose and added sugars (39, 40). The diet’s fiber content helps promote the growth of bacteria that produce short-chain fatty acids with anti-inflammatory properties, thereby reducing fat accumulation in the liver through their metabolism (41, 42). Furthermore, since fructose accelerates fat accumulation in the liver by increasing lipogenesis and fat oxidation, reducing added sugar intake—especially fructose—may significantly lower liver fat accumulation (43, 44). The Mediterranean diet also restricts saturated fat intake by limiting red and processed meats, further reducing liver fat accumulation, while balanced consumption of poultry and fish provides healthier alternatives (45). Overall, the Mediterranean diet represents a healthy dietary pattern that contrasts with the “Western diet,” which has been reported to increase the risk of NAFLD in Hassani Zadeh's meta-analysis (25).
This umbrella review had several limitations. First, although the systematic search was conducted without restrictions, the potential for missed unpublished reports and archived records cannot be ruled out. Additionally, the number of meta-analyses on prospective studies was limited, and the long-term effects of dietary patterns could not be fully assessed. Therefore, future meta-analyses should focus on more prospective studies to better understand the long-term effects of specific dietary patterns on NAFLD.
5.1. Conclusions
The findings of this umbrella review summarize, for the first time, meta-analyses on dietary patterns and NAFLD. The calorie-restricted diet demonstrated the most significant improvements in NAFLD-related outcomes. Based on these findings, the Mediterranean diet can also be considered an effective treatment approach for NAFLD patients.