1. Background
Vitiligo is a commonly acquired skin disease in which the active melanocytes are destroyed.
Vitiligo's prevalence makes up 2 - 5% of the population worldwide, and its average age is 20 years in all races in all countries (1).
Different genetic and environmental factors create Vitiligo; a positive family history has been reported in one-third of people, which shows that genetics plays a vital role in this disease. Many theories have been raised about the creation of this disease, but none of them have been accepted. The theories with the highest studies are described in the following (2).
In autoimmunity theory, the autoimmunity factor is significant in creating Vitiligo (3).
Investigating the Vitiligo lesions with immunohistochemistry shows evidence of extant inflammatory cells, with an increasing rate of CD8+ cells to CD4+, which indicates the destruction of melanocytes with CD8+ T-cell lymphocytes (4). Increasing the free radicals causes cell damage and, eventually, cell destruction when melanocytes pass the cell walls (5, 6), reducing the production of TNF-α, IL-2, and IL-6. Research has advocated that statins possess strong protection against oxidative damage by removing free radicals and retrieving enzymatic and non-enzymatic antioxidant systems (7). Statins reduce the ratio of TH1/TH2 and CD4/CD8 and the activation of the nuclear factor Kappa B (NF-κB) pathway during oxidative stress and create a balance between cytokines produced by type 1 helper (TH1)/type 2 helper (TH2) cells (8).
Prostaglandin E2 (PGE2) increases the rate of melanogenesis and accounts for a large portion of UVA therapeutic effects in treating vitiligo. In addition, cAMP triggers both melanogenesis and melanocyte stem cell proliferation. cAMP further activates glucose-6-phosphate dehydrogenase (G6PD), a key antioxidant whose concentration is reduced in vitiligo. Research has shown that simvastatin raises the level of cAMP and exhibits gastroprotective effects by removing free radicals and increasing the levels of nitric oxide (NO) and PGE2 (9). As a result, statins can be used as part of anti-vitiligo treatment plans since dominant TH1, IL-6, NO, H2O2, oxidative stress, and immune-modulating effects play a key role in the pathogenesis of vitiligo, and statins trigger the production of melanocytes. Statins (e.g., simvastatin, atorvastatin, etc.), particularly simvastatin, exhibit various anti-inflammatory effects such as producing immune-modulating markers (e.g., TGF-β, IL-10, etc.) and r Simvastatin is a lipid-lowering drug. This drug is a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-COA), determining the cholesterol synthesis rate. The HMG-COA reductase inhibitors reduce the concentration of the whole cholesterol, LDL, and very low density lipoprotein (VLDL) in plasma and cause the triglycerides (TG) to decrease (10).
2. Methods
The protocol of this study was approved by the Ethics Committee of Shiraz University of Medical Sciences (ethics code: IR.SUMS.MED.REC.1395.20).
In this study, an intervention was performed as a double-blind, randomized clinical trial; 50 people with generalized Vitiligo (based on the clinical investigation and Wood's lamp examination) were referred to the dermatologic clinic of the Shahid Faghihi Hospital, Shiraz, who had the light-therapy indication in an Narrowband_ ultraviolet B (NB_UVB) method, investigated in 2015. The inclusion criteria were non-diabetic patients without thyroid and liver diseases, ones over 14 years old, women who accept reliable contraception methods, those who have not used the oral or local Vitiligo drug for four weeks, and those who signed an informed consent form.
The exclusion criteria included liver, kidney, and thyroid problems; pregnant and lactating women, contraindication for light therapy; patients under 14 years, ones suffering from simvastatin lesions such as cramps, muscle pain, and liver problems, headache, kidney problems, dark urine, and digestive complications, receiving another treatment within the recent four weeks, and the cases taking a simvastatin-interfered drug (azole antifungal, cyclosporine, erythromycin, clarithromycin, danazol, gemfibrozil, diltiazem, verapamil, amlodipine... due to increased risk of myopathy).
As a first step, the resident supervisor determined the intensity of the disease using the Vitiligo Area Severity Index (VASI) so that each lesion was considered a unit of the same size as a palm, based on its size. As shown in Figure 1, the percentage of depigmentation was multiplied by each unit, and the total lesion scores were obtained using following equation:
This process was conducted at each examination. The level of lipids, liver enzymes, Calvin-Benson cycle (CBC), Blood urea nitrogen (BUN), and the creatinine of patients was determined, and the involved site was photographed. Then, they randomly fell into one of two groups under light therapy of Narrowband ultraviolet B phototherapy (NBUVB) among with placebo in a block (made by School of Pharmacy, Shiraz University of Medical Sciences; first month twice a day, second, three times a day, and third and fourth, two tablets twice a day) or the light therapy with 20 mg simvastatin (made by Pursina, Iran, Tehran; first month twice a day, second, three times a day, and third and fourth, two tablets twice a day – i.e. 80 mg per day and the highest dosage can be prescribed in hyperlipidemic patients in the specific conditions), and the amount of reducing score of patients' lesions was studied by VASI. In this criterion, the percentage of returns of pigments is categorized into six groups (11).
The NB_UVB light therapy was to begin three times a week for each patient in a cabin (with a Waldman device; UV5040BL model) of the light therapy part of Shahid Faghihi Hospital) with 100 mJ/cm2 and increased its dosage up to 10 mJ/cm2 based on the patient's skin reaction. The patients visited and were followed during this time monthly by a weekly SMS to take the medication. The photography (with a Canon. full HD. X50 Japan) and scoring were performed until the end of this study. The tests were examined basically and at the end. The clinic secretariat provided Simvastatin and placebo packages in similar forms with A and B codes, and an uninformed person (resident) evaluated clinical results. The lateral complications (incident of muscular pains or muscular weakness) and tests, at first and the end, were explored by a person other than the evaluator of clinical results and VASI in two groups (due to informing the possibility of intervention after observation of reduced amount of lipid in those who received simvastatin). Light therapy or simvastatin can cause any severe signs or other severe changes in experiments/patients' requirements, leading to the patient being excluded from the study and receiving the popular and proper treatment. Finally, the A and B codes were decoded.
3.1. Information Analysis Method
The collected data were analyzed using SPSS software version 13, in which frequency and percentage were calculated for qualitative variables, and averages and standard deviations (STD) were calculated for quantitative variables. The statistical tests were chi-square, independent t-tests, and paired t-tests, and the significance level for interpreting the results was 0.05.
4. Results
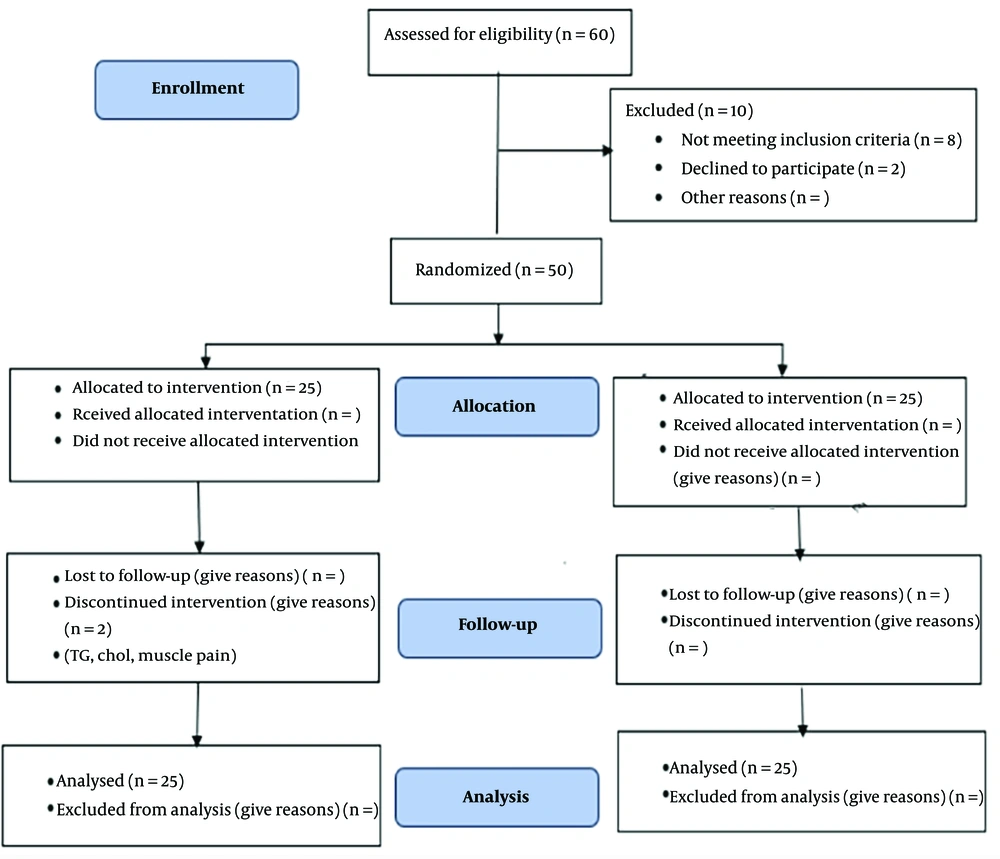
In the beginning, about 62 patients were visited, and about eight were excluded due to lack of necessary criteria and two due to dissatisfaction; 50 were divided into two groups, where one person did not continue due to decreased TG and cholesterol. One, due to muscular pains, was left regardless of their normal tests, and two people were substituted for it (Figure 2).
The number of qualified persons in this study was based on the inclusion and exclusion characteristics: Fifty patients with generalized Vitiligo in the age range of 20 - 60 years and an average age of 41 ± 19 divided into two random groups. The patients do not differ in average age, gender, onset and duration of disease, history of the family, history of autoimmune disease in the patient, infected anatomical site, and other statistical parameters related to this disease (P > 0.05).
Table 1 compares the therapeutic effect of NBUVB and placebo with NBUVB and oral simvastatin separately in the monthly visit, examination, and total. Table 2 presents the results of preclinical experiments in two groups.
| Variables | Mean | STD | P-Value |
|---|---|---|---|
| First score | 0.982 | ||
| NBUVB + simvastatin | 9.855 ± 11.378 | 11.377 | |
| NBUVB + placebo | 9.287 ± 11.348 | 11.348 | |
| Second score | 0.046 | ||
| NBUVB + simvastatin | 4.483 ± 4.249 | 4.249 | |
| NBUVB + placebo | 9.423 ± 11.074 | 11.074 | |
| Third score | 0.020 | ||
| NBUVB + simvastatin | 3.732 ± 2.959 | 2.959 | |
| NBUVB + placebo | 8.367 ± 10.804 | 10.804 | |
| Fourth score | 0.021 | ||
| NBUVB + simvastatin | 2.575 ± 2.616 | 2.616 | |
| NBUVB + placebo | 8.310 ± 10.796 | 10.697 | |
| Fifth score | 0.015 | ||
| NBUVB + simvastatin | 1.537 ± 1.812 | 1.812 | |
| NBUVB + placebo | 7.233 ± 10.245 | 10.542 | |
| Variables | NBUVB + Simvastatin | NBUVB + Placebo | P-Value |
| Age | 41.04 ± 19.02 | 40.88 ± 19.10 | 0.903 |
| Onset of disease | 35 ± 21.08 | 35.16 ± 21.02 | 0.845 |
| Male: Female ratio | 18.7 | 17.8 | 0.720 |
| Family history of positive vitiligo | 8 (32) | 7 (28) | 0.957 |
| Family history of autoimmune disease | 12 (48) | 13 (52) | 0.763 |
| Site involvement | |||
| Head and neck | 15 | 19 | 0.762 |
| Extremities | 19 | 17 | 0.752 |
| Trunk | 20 | 21 | 0.345 |
| Other site involvement | 2 | 4 | 0.412 |
a Values are expressed as No. (%) or mean ± SD unless otherwise indicated.
| Variables | Before | After | P-Value |
|---|---|---|---|
| Leukocyte | 0.022 | ||
| NBUVB + simvastatin | 5083.3 ± 868.4 | 5440 ± 805 | |
| NBUVB + placebo | 5200 ± 775.7 | 4737.5 ± 1532.2 | |
| Hemoglobin | 0.312 | ||
| NBUVB + simvastatin | 13.4 ± 2 | 13.3 ± 1.6 | |
| NBUVB + placebo | 13.5 ± 2 | 13.4 ± 2 | |
| Platelet | 0.205 | ||
| NBUVB + simvastatin | 256875 ± 59626.5 | 256615.4 ± 32133.9 | |
| NBUVB + placebo | 257125 ± 59457.4 | 256875 ± 59550.4 | |
| Triglyceride | 0.013 | ||
| NBUVB + simvastatin | 151.8 ± 88.9 | 102.1 ± 49.2 | |
| NBUVB + placebo | 154.5 ± 49.5 | 150.9 ± 48.1 | |
| Total cholesterol | 0.027 | ||
| NBUVB + simvastatin | 162.8 ± 48 | 103.2 ± 59.6 | |
| NBUVB + placebo | 156.5 ± 63.1 | 154.5 ± 60.8 | |
| LDL | 0.112 | ||
| NBUVB + simvastatin | 99.8 ± 61.9 | 86.9 ± 57.8 | |
| NBUVB + placebo | 107.1 ± 60.3 | 102.5 ± 60.9 | |
| ALT | 0.258 | ||
| NBUVB + simvastatin | 21.3 ± 10.5 | 23.7 ± 14.7 | |
| NBUVB + placebo | 21.5 ± 10.5 | 21.8 ± 10.5 | |
| AST | 0.213 | ||
| NBUVB + simvastatin | 19.3 ± 5.6 | 20.9 ± 7.3 | |
| NBUVB + placebo | 19.3 ± 5.6 | 19.5 ± 5.5 | |
| BUN | 0.158 | ||
| NBUVB + simvastatin | 10.8 ± 2.5 | 11.3 ± 1.1 | |
| NBUVB + placebo | 10.7 ± 2.5 | 10.6 ± 2.5 | |
| Creatinine | 0.094 | ||
| NBUVB + simvastatin | 0.9 ± 0.2 | 0.9 ± 0.1 | |
| NBUVB + placebo | 0.9 ± 0.2 | 0.9 ± 0.2 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The simvastatin added to phototherapy was investigated as a standard treatment, which is similar to photochemotherapy and excimer laser (12) in terms of the therapeutic effect of meta-analysis.
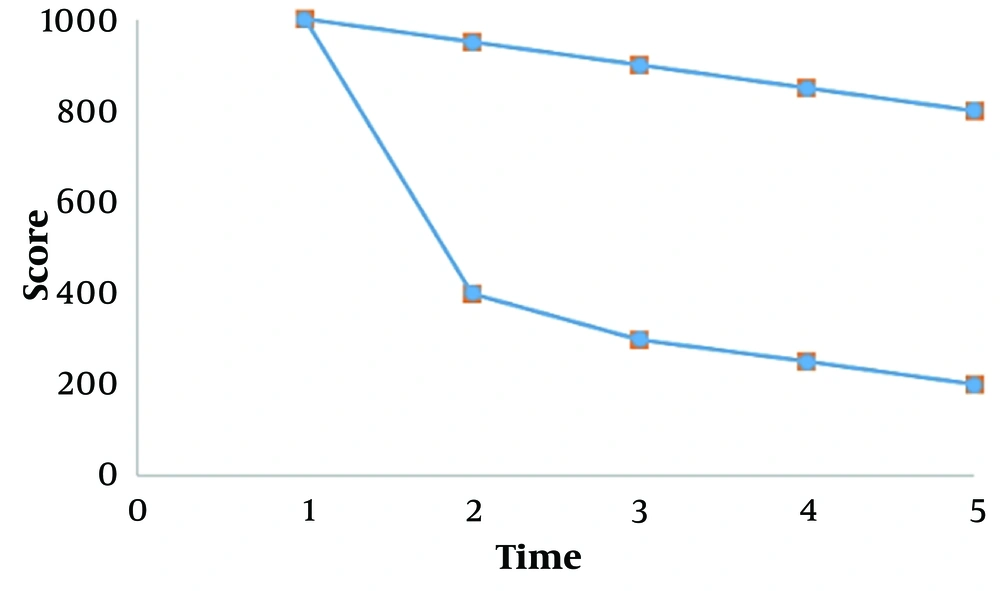
Compared to the first study, this study had the same mean disease intensity score in both simvastatin and placebo groups; the study was a double-blind, randomized clinical trial on the acceptable number of Vitiligo patients (P > 0.05). Then, the disease intensity decreased significantly more in the simvastatin group than in the placebo group after that, with no lateral clinical or experimental complications (P = 0.0001).
As observed in Table 1, the mean score of disease intensity was calculated first in two control groups of simvastatin and placebo using an independent t-statistical method. It was the same (P > 0.05) but significantly reduced in the simvastatin group more than the placebo group after (P = 0.015).
The patients of the two groups do not differ in terms of mean age, gender, beginning age, duration of disease, family history of vitiligo, history of autoimmune infection, infected anatomical site, and other statistical parameters using the chi-square method (P > 0.05).
As observed in Figure 1, the mean score of disease intensity is similar at the beginning of the study in two groups of simvastatin and placebo (P > 0.05), but after that, the score of disease intensity was significantly reduced in the simvastatin group more than the placebo (P = 0.0001)
5. Discussion
Oral simvastatin enhances the photothermal outcomes while exerting trivial side effects. Thus, combining oral simvastatin with phototherapy is recommended as a potent strategy for treating patients with vitiligo.
In a case study by Noel et al. from Canada, a 50-year-old man with Vitiligo and hypercholesterolemia showed that simvastatin with an 80-mg dosage could improve the depigmented lesions, and any other complication was not observed in the patient. After a washout, the patient is placed on atorvastatin to confirm the simvastatin effect. The patients experienced a headache after two weeks, and their Vitiligo lesions intensified; serial photos of the patient after continuous Simvastatin use revealed that the Vitiligo-lesion severity improved after a year (13). In this study, this good effectiveness was observed with low complications.
In the last publication indexed by PubMed, Agrawal et al. During a large experiment conducted on mice infected by Vitiligo and undertreated in the US with simvastatin injections, this drug effectively treated depigmentation in the ears, nose, foot soles, and tail regions. The drug effects were described as reduced CD8+ proliferation and Gamma-interferon production in an animal sample injected three times a week for five weeks (14). The exact molecular underlying mechanisms can also induce this good effectiveness, as observed in the present study.
The first subjects in this field were announced in a US review study by Kobashigawa. They said that the HMG-COA-reductase inhibitory drugs such as simvastatin can effectively improve autoimmune diseases like Vitiligo due to their immunosuppressive effects (15). Another review study by Namazi in Shiraz announced that statins, especially simvastatin, create significant changes in the immune system due to their effects on macrophages and T-helper lymphocytes, as well as with induction of inflammatory path and production of NO, which can be effective in improving skin diseases with autoimmne origin such as Vitiligo (16).
Even though light therapy has been raised as an effective therapeutic method for Vitiligo, the long-term and referral necessity of treatment centers requires to be followed to find a method for its known complications to increase its impact. Despite some extent targeted local drugs, there is not an effective systematic treatment that can be used alone or along with light therapy in patients with Vitiligo; the impact of some drugs, such as systematic corticosteroids with different regimes (12), antioxidants such as vitamin E and minocycline have not been helpful and can be raised practically in guidelines. As a result of the lack of certainty regarding the pathogenesis of diseases and the critical role that the immune system plays in them, the new articles suggest that research and investigation should focus on low-complication immunosuppressive drugs such as methotrexate, cyclosporine, and melanocyte stimulating hormone (MSH). Compounds such as afamelanotide ([Nle4-D-Phe7]-alpha-MSH), management, and sometimes hopeless assistance with mental support for patients, even in monotherapy forms, as well as stem cells and gene therapy (17). Thus, the current study on low-complication and cheap drugs like simvastatin can be the onset of more efforts to assist patients and future research. Furthermore, multicenter studies with higher sample volume, high treatment duration, and time tracking after leaving the treatment for the return of depigmentation are strongly recommended.
5.1. Conclusions
Based on the results, it is inferred that oral simvastatin can efficiently treat the skin lesions of Vitiligo and does not have many lateral complications, and its use with light therapy in those with Vitiligo is recommended. Finally, multicenter studies were proposed with higher sample volume, high treatment duration, time tracking after leaving the treatment for return of depigmentation, investigation of patients with Dermatology Life Quality Index (DLQI) criterion, the effect of simvastatin on the decrease in the number of phototherapy sessions, use of other newer statins, comparison with different results of therapeutic methods, or others in sufferers with Vitiligo.
According to related laboratory investigations, the CBC changes, liver enzymes, and kidney performance tests were investigated in two groups using the same paired t-statistical method. Triglycerides and cholesterol were reduced in the simvastatin group.