1. Background
Stroke is one of the leading causes of permanent disability in today's advanced societies, which imposes short-term and long-term costs on the family, society, and the medical system of countries (1). Spasticity is one of the most common clinical symptoms explored after stroke. About 30% to 40% of people experience spasticity after a stroke (2). Spasticity occurs as a result of changes in the mechanical properties of the muscle and the reflex properties following upper motor neuron syndromes (3). Patients with stroke often face functional mobility limitations because of spasticity, which may improve some functional movements. For example, spastic ankle affect negatively on walking performance (4). In some studies, EMG has been employed to quantify the response stimulated by either the muscle (M-reflex) or electrical stimulation of the peripheral nerves innervating the muscle (H-reflex) in spastic patients where responses are exaggerated and related to the extent of spasticity (5).
Restoring upper and lower limb function is one of the most essential rehabilitation goals for people with central nervous system diseases. Both active and passive rehabilitation interventions improve strength and flexibility. Studies have reported that patients with central nervous system diseases perceive rehabilitation protocols as repetitive, long-term, and monotonous, which decreases the motivation and participation of patients to participate in rehabilitation treatment programs (6). In recent years, the use of virtual reality-based treatments has increased as one of the types of interventions in neurorehabilitation (7). Virtual reality allows patients to interact with simulated environments that are similar to real environments (8). Among the various virtual reality devices, non-immersive virtual reality devices are the best for integration into rehabilitation treatment programs. Accordingly, the Nintendo Wii is one of the most widely used virtual reality devices for neurological diseases, including known strokes (9). Nintendo Wii requires the participant to actively participate in various sports and recreational activities, such as games, while standing or sitting and can be adapted to the patient's needs (10). Nintendo Wii is a valuable tool that provides flexibility, balance, strength, and coordination training in safe environments such as homes or clinical centers, which can be used to train individuals in a targeted manner (10). Many researchers have conducted studies on the effect of Nintendo Wii in improving upper limb function and balance (11). Saposnik et al. investigated the safety and practicality of using Nintendo Wii to facilitate the upper limb function of patients with Stroke (12). Studies have also described Nintendo Wii exercises improving balance, performing daily activities, and walking efficiently (13). Meanwhile, Yatar and Yildirim found that despite the effectiveness of this method, there was no significant difference between the Nintendo Wii and the Bobat method in treating patients with chronic stroke (14).
In previous articles, the positive effect of using Nintendo Wii has been proven to improve the balance and movement performance of the upper and lower limbs of patients with stroke. However, there is no evidence of the effect of this intervention in reducing or increasing spasticity in patients with stroke.
2. Objectives
This clinical trial study aimed to compare the effectiveness of conventional physiotherapy alone with the combination of conventional physiotherapy and the use of the Nintendo Wii game console on the outcomes of spasticity in patients with stroke. The authors of this article assume that adding Nintendo Wii to conventional physiotherapy can significantly reduce the spasticity of the ankle plantar flexor muscles in patients with stroke.
3. Methods
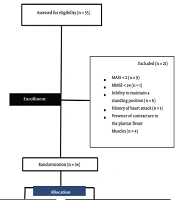
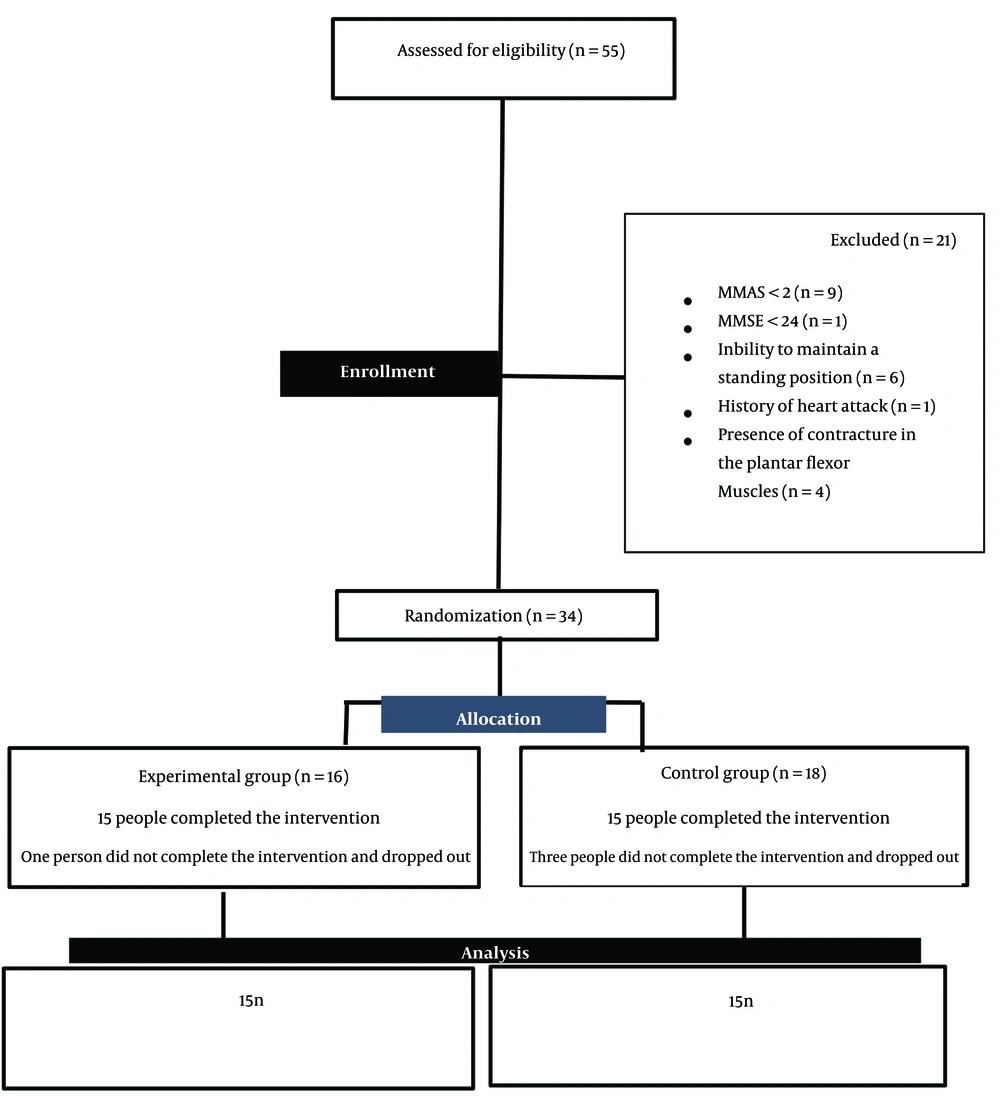
The present study is a parallel single-blind randomized clinical trial in which patients are randomly assigned to one of the two groups of conventional physiotherapy alone (control group) or the combination of conventional physiotherapy and games with a Nintendo Wii console (experimental group). The target population was male and female with stroke. In the present study, the sampling method was convincing and available. All stages of the current research, including clinical evaluations and treatment sessions, were conducted in the physiotherapy clinic of Ayatollah Kashani Hospital in Isfahan city between July and December 2022. Patients were assigned to two groups based on the random block method by the clinic secretary, who was unaware of the study. Using the appropriate formula and based on a survey (15) and considering the significance level α = 0.05 and Z = 1.96, the statistical power = 80% (Z1-β = 0.84) and the sample size of 15 people in each group were estimated. The protocol of this study was approved by the Ethics Committee of Isfahan University of Medical Sciences (ethic code: IR.MUI.RESEARCH.REC.1399.309) and prospectively registered in the Clinical Trials Center of Iran (IRCT code: IRCT20200101045970N3). The flowchart related to the number of people present in each stage of the study is shown in Figure 1. As reported in a flowchart, 55 people with stroke were evaluated, and 21 were excluded from the survey for reasons such as lack of entry criteria and unwillingness to participate. A total of 34 patients with stroke were randomly assigned to one of the control and experimental groups. During the treatment period, a total of four eligible people did not complete the treatment period. Finally, the data obtained from 30 stroke patients who completed the treatment course were used for statistical analysis. The criteria for entering the study include age 18 to 85 years (16), the presence of a history of the first unilateral stroke that has been proven by a neurologist diagnosis through CT scan or MRI findings (17), the presence of spasticity in the ankle joint two or more. According to the Modified Modified Ashworth Scale (MMAS) criterion (18), the presence of appropriate cognitive status based on the Persian version of the mini-mental status exam (MMSE) was > 24 (3), and the ability to maintain a standing position without using aids for at least 30 seconds (19). In addition, exclusion criteria included a history of heart attack or high-risk heart disease (18), the presence of other neurological disorders such as neuropathy, epilepsy, convulsions (19), the use of botulinum injection or other antispastic drugs (4), participation in other physiotherapy interventions, (3) and the presence of contracture in the plantar flexor muscles (4).
3.1. Outcomes Measure
The outcomes were the clinical outcome of spasticity and neural properties. The study outcomes were evaluated in two stages: Before the start of the treatment and at the end of the treatment period. This study assessed the clinical outcome of the spasticity level of ankle plantar flexor muscles using the MMAS. This is a common and reliable tool for measuring the degree and amount of spasticity, which evaluates the amount of resistance to passive stretching of the involved muscles (20). The examiner, unaware of the groups, determines the degree of resistance to passive movement from 0 to 4 to determine the degree of spasticity based on the Persian version of the MMAS (21).
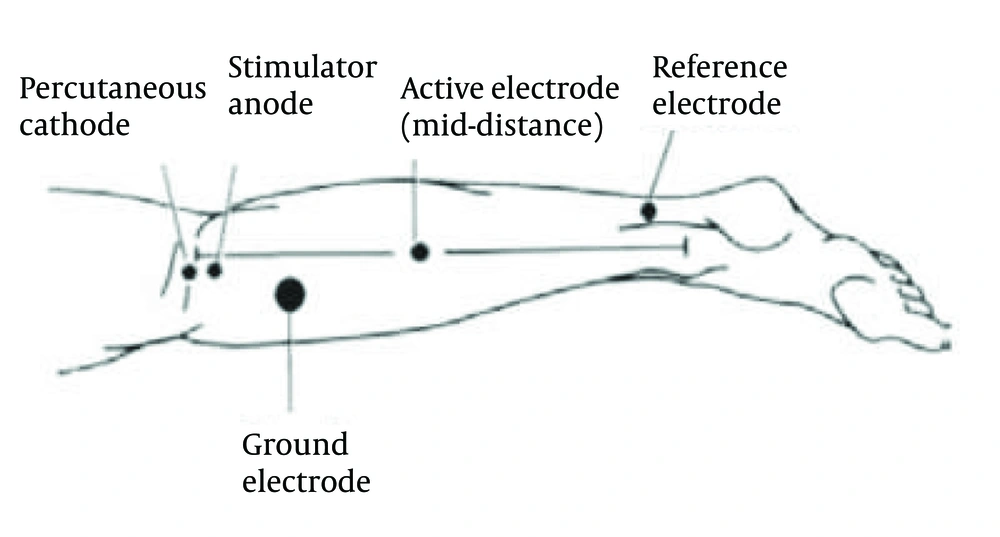
Neural properties, including H-reflex amplitude, H-reflex delay time, M wave amplitude, and the ratio of H-reflex amplitude to M wave amplitude, were measured in this study. Neural parameters were measured by an experienced neurologist unaware of the study subjects and treatment groups. Patients were asked to sleep on the bed with their feet hanging over the edge of the bed. Figure 2 illustrates how the tibial nerve is stimulated in the popliteal cavity after preparing the skin. The stability electrode is placed on the gastrocnemius muscle between the medial malleolus and medial epicondyle of the tibia, and the grand electrode is placed between the stimulating and receiving electrodes. Nerve stimulation was performed according to the Braddom and Johnson method with 1ms diversion and a frequency of one every 5s, and then electrophysiological indicators including the H-reflex amplitude, H-reflex delay time, M wave amplitude, the ratio of the H-reflex amplitude to the M wave amplitude in this study were recorded (22).
3.2. Treatment Protocol
Both groups were treated for 12 sessions four weeks after initial evaluations. Both groups received the therapeutic exercise protocol under the supervision of a physiotherapist three times a week for four consecutive weeks and a total of 12 sessions (23). Furthermore, the experimental group also used the Nintendo Wii console three times a week for four consecutive weeks and 12 sessions in addition to therapeutic exercise. The games were played using the Nintendo Wii console while the patient stood on the balance board. The patients had already received the necessary training from the physiotherapist and were controlled by him during the game. In each session, three out of five games were selected based on the participation of the physiotherapist's opinion and the patient's preference. People were at a distance of three meters from the screen. Each session lost 30 minutes.
3.3. Data Analysis Method
All statistical analyses were performed using (IBM Statistical Package for the Social Sciences) SPSS version 20. The Shapiro-Wilk test was used to evaluate the distribution of data. Mann-Whitney test was used for inter-group comparison of spasticity degree, and Wilcoxon test was used for intra-group comparison. An independent t-test was used to compare two groups before starting treatment for other outcomes with normal distribution. One-way analysis of variance/covariance "ANCOVA/one-way ANOVA" was used to compare the two groups after the start of the study. In the variance/covariance analysis, the results were reported once without controlling the values before the treatment (raw) and once with the control of the values before the treatment (corrected). In this study, the final model is the corrected model. Paired t-test was used for intragroup comparisons. Cohen's d effect size was used to examine the magnitude of intergroup differences and determine the effectiveness of the test (using the Nintendo Wii console). The level of significance in the present study was considered 0.05.
4. Results
A total of 30 stroke patients with lower limb spasticity completed this clinical trial study. Table 1 shows the demographic variables of both groups. As reported in Table 1, the average age of the participants in the experimental group is 57.07 years, and the average age in the control group is 60.33 years. In addition, there was no statistically significant difference between the two groups in quantitative background variables (P > 0.05).
| Groups | Age (y) | Weight (kg) | Height (cm) | Duration of Disease (mo) | Stroke Type | Stroke Side | Gender |
|---|---|---|---|---|---|---|---|
| Experimental | 57.07 ± 12.21 | 66.80 ± 9.89 | 169 ± 8.24 | 17.33 ± 13.15 | H = 5, I = 10 | R = 9, L = 6 | M = 11, F = 4 |
| Control | 60.33 ± 12.97 | 67.60 ± 10.43 | 164.53 ± 9.27 | 25.73 ± 13.53 | H = 5, I = 10 | R = 9, L = 6 | M = 8, F = 7 |
Abbreviations: H, hemorrhagic; I, ischemic; R, right; L, left; M, male; F, female.
Descriptive and analytical statistics of primary and secondary outcomes are presented in Table 2. The median intensity of spasticity before the start of treatment in the test and control groups was 3 and 2, respectively. After the end of the treatment, the median intensity of spasticity in both groups was 2. In the analytical statistics section, except for the result of spasticity degree, which did not have a normal distribution and non-parametric tests were used, other results had a normal distribution.
| Variables | Experimental Group (n = 15) | Control Group, (n = 15) | Between Two Groups | Within the Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P-Value | z Index/f Index | Cohen's d | P-Value | ||||||||
| Baseline | After Intervention | Baseline | After Intervention | Baseline | After Intervention | Baseline | After Intervention | Experimental | Control | ||
| Spasticity level (score) | 3 (1) | 2 (1) | 2 (1) | 2 (1) | 0.72 | 0.21 | -0.36 | -1.26 | - | 0.001 | 0.096 |
| H-reflex latency (ms) | 31.66 (4.93) | 31.32 (3.74) | 31.35 (4.5) | 30.83 (3.18) | 0.85 | 0.7 a, 0.69 b | 0.18 | 0.15 a, 0.17 b | 0.14 a, 0.15 b | 0.64 | 0.45 |
| The maximum amplitude of the H-reflex (mv) | 4.53 (2.45) | 4.21 (2.26) | 3.29 (1.35) | 3.55 (1.27) | 0.1 | 0.33 a, 0.66 b | 1.71 | 0.97 a, 0.19 b | 0.36 a, -0.16 b | 0.64 | 0.48 |
| The maximum M-wave amplitude (mv) | 13.13 (4.93) | 11.88 (3.97) | 11.34 (4.26) | 11.99 (5.30) | 0.3 | 0.95 a, 0.82 b | 1.06 | 0.1 a, 0.37 b | -0.03 a, -0.33 b | 0.25 | 0.54 |
| The ratio of the, H max / M max | 0.37 (0.18) | 0.36 (0.18) | 0.34 (0.2) | 0.34 (0.14) | 0.84 | 0.77 a, 0.84 b | 0.21 | 0.09 a, 0.04 b | 0.11 a, 0.08 b | 0.97 | 0.9 |
a Raw model.
b Corrected model.
4.1. Intergroup and Intragroup Analysis of the Degree of Spasticity
Before the treatment, there was no significant difference in the mean of spasticity between the two groups (P > 0.05). In addition, the comparison of the average degree of spasticity between the two groups after treatment was not significant (P > 0.05). Comparing the degree of spasticity before and after the treatment within each group showed that in the experimental group, the degree of spasticity decreased significantly (P < 0.05). In contrast, in the control group, no significant difference was observed (P > 0.05).
4.2. Intergroup and Intragroup Analysis of H-reflex Latency
There was no significant difference in the average H-reflex latency between the two groups before the treatment (P > 0.05). The results of one-way analysis of variance/covariance showed no significant difference in the delay time of the H-reflex between the two groups with and without controlling the baseline values after the end of the treatment period (P > 0.05). Based on the Cohen-Dham Effect Size Index, none of the two treatment methods had a significant advantage over the change in the delay time of the H reflex, and the magnitude of the difference between groups for this outcome was insignificant. The results of intragroup analysis showed no significant change in the delay time of the H-reflex in both the experimental and control groups after the treatment, compared to before the treatment (P > 0.05).
4.3. Intergroup and Intragroup Analysis of the Ratio of the Maximum H-reflex Amplitude to the M Wave Amplitude
Before the treatment, no significant difference was observed in the ratio of the H-reflex amplitude to the M wave amplitude between the two groups (P > 0.05). Based on the one-way analysis of variance/covariance with and without control of baseline values, there was no significant difference in the ratio of the H-reflex amplitude to the M wave amplitude between the two groups after the end of the treatment period (P > 0.05). According to Cohen's Effect Size Index, the magnitude of the difference between groups for this outcome was insignificant and insignificant. In the control and experimental groups, there was no significant difference in the ratio of the H-reflex amplitude to the M wave amplitude before and after the treatment (P > 0.05).
5. Discussion
This study showed a significant difference in the clinical outcome of spasticity in the experimental group compared to before the treatment. In contrast, in the control group, there was no significant difference between before and after the treatment. After treatment, the two groups had no significant difference in the clinical outcome of spasticity. Moreover, a comparison between control and experimental groups in this study revealed that neither had a significant change in H-reflex latency or ratio of H-reflex amplitude to M wave after the end of the treatment period, compared to before the treatment period. There was no significant difference between groups.
Spasticity after a stroke can cause pain, impaired hand movements, limited range of motion, impaired walking and daily activities, and finally, a severe decrease in the quality of life in stroke patients (24, 25). There are many pharmacological and non-pharmacological therapeutic interventions aimed at reducing spasticity and improving function in stroke patients. Among conservative treatments, acupuncture, and dry needling can be mentioned (26). Exercise therapy is a key and cheap treatment for reducing muscle tone and improving function in people with a history of stroke. In a study, Park et al. investigated the effectiveness of therapeutic exercise and electrical stimulation (15). Exercise therapy included floor exercises and walking for thirty minutes in each session. Following the combination of electrical stimulation and exercise therapy, a significant reduction in the level of spasticity was observed compared to the exercise therapy group alone (15). Zhang et al. showed that therapeutic exercise in water is more effective than usual therapeutic exercise in improving function, but no difference was observed between the two groups in the degree of spasticity (27). The most common and widely used conservative treatment test for increasing the range of motion and managing spasticity is stretching exercises, which are prescribed as an essential component in rehabilitation and treatment protocols (28). In the present study, the control and experimental groups received gastrocnemius muscle stretching and were walked on a parallel bar.
Several studies have investigated the effects of using the Nintendo Wii on improving outcomes related to central nervous system diseases, including spasticity. In Gatica-Rojas et al., the use of the Nintendo Wii game console for six weeks in children with spastic cerebral palsy, compared to before treatment, led to a significant reduction in the degree of spasticity and improved balance based on the decrease in pressure center fluctuations (29). In Atasavun Uysal and Baltaci, Nintendo Wii and conventional physiotherapy, including balance and posture correction exercises, were compared to conventional physiotherapy for children with hemiplegic cerebral palsy. Nintendo Wii can be an effective method for improving balance in children with cerebral palsy; however, there was no significant preference for Nintendo Wii over conventional interventions in other outcomes (30).
In this study, electrophysiological indicators were also used to evaluate the effectiveness of Nintendo Wii on neural properties and clinical outcomes. Among the many reflexes obtained by electrical stimulation, the H-reflex is better known than the others. H-reflex latency and Hmax/Mmax ratio are reliable indices to measure α motor neuron excitability (31). Several studies have demonstrated an increase in the ratio of the H-reflex to stretch reflex of the muscle (H/M ratio) in people affected by stroke in comparison to healthy people (5, 32). This ratio shows the readiness and excitability of motor neuron pools, which increases in spasticity. In this way, the amplitude of the H-reflex rises, and the amplitude of the M wave decreases (33). An increase in loop gain of the monosynaptic arc, enlarged excitation in postsynaptic afferents from descending pathways, or a decrease in presynaptic inhibition can account for this effect (32, 34).
In the present study, no significant change in H-reflex latency and the ratio of H-reflex amplitude to M wave amplitude was observed in any of the two groups of conventional treatment alone and the combination of conventional treatment and Nintendo Wii. In addition, the comparison between groups did not show a significant change. In other studies, no significant change was observed in the H-reflex latency and amplitude (22). Bakheit et al., for instance, suggested that a single session of isotonic and isokinetic stretching did not significantly affect the Hmax/Mmax ratio and H-reflex latency (22). According to the present study, there was no significant difference in neurological variables because both groups of people with stroke were in the chronic stage of the disease for an extended period of time since the stroke had occurred. At this time, recovery in the nervous system is minimized. A study showed that stroke patients could be divided into two groups in terms of neurological and mechanical factors: The larger group (seven out of 11 people) named Stroke with Low Reflex Torque (SLRT), and the smaller group named stroke with high reflex torque (SHRT) was divided (35). In hemiplegic spastic patients, the degree of facilitation of Ia fibers was similar to that of the control group during the H-reflex of the soleus muscle, indicating that presynaptic inhibition does not change in these patients. Therefore, stroke patients were expected to show reflex stiffness similar to the control group's.
The limitations of the present study were the small number of samples, the number of treatment sessions, and the lack of follow-up, which were not considered in this trial due to time constraints. Other limitations of the study included the lack of assessment of adherence and the degree of acceptability of the treatment by the participants.
5.1. Conclusions
The results showed that the clinical outcome of spasticity was significantly reduced after the end of the treatment in the Nintendo Wii group. Compared to conventional treatment alone, intergroup comparisons did not show a significant advantage in using Nintendo Wii for spasticity outcomes.