1. Background
Peptic ulcers are erosions caused by discontinuation or disruption in the inner lining of the gastrointestinal tract (GIT) due to hyperacidity, which often occurs in the stomach with the first and second parts of the duodenum. However, peptic ulcers are not limited to these areas, and they can involve the lower esophagus, end of the duodenum, or jejunum (1). Peptic ulcers are responsible for the variable of symptoms, including nausea, vomiting, abdominal pain, weight loss, bleeding, and perforation, which may occur in the severe form of the disease (1).
Various factors contribute to the development of peptic ulcers, including Helicobacter pylori infection (2), nonsteroidal anti-inflammatory drug (NSAID) and oral steroid use, and tobacco consumption (3-5). Helicobacter pylori infection and NSAID account for most cases of peptic ulcer disease (2, 6, 7). Peptic ulcers, which involve gastric and duodenal ulcers, usually are not differentiated based on history alone, but epigastric pain is the most common symptom in both, characterized by a burning sensation, which occurs 2 - 3 hours after a meal in duodenal ulcer and shortly (15 - 30 minutes) after a meal in gastric ulcer. Helicobacter pylori is responsible for 70 - 90% of gastric ulcers and 90% of duodenal ulcers, which can colonize the gastric mucosa, cause inflammation, and impair the secreting of bicarbonate to enhance the development of gastric acidity, which is more common in lower socioeconomic status and often acquires during childhood (8).
According to a prospective study in Taiwan, of patients who undergo upper gastro-intestinal (GI) endoscopy as a routine health assessment, nearly two-thirds of them who had peptic ulcer were asymptomatic (9). A detailed history of NSAID use and H. pylori infection is essential. Upper endoscopy could be used for the diagnosis of peptic ulcer disease, and it is necessary for those who have dyspepsia and alarm symptoms such as weight loss, age > 60 years, family history of gastrointestinal tract (GIT) malignancy, dysphagia, early satiety, GIT bleeding, vomiting, Iron deficiency anemia) endoscopy to take a biopsy for peptic ulcer allows to distinguish benign and malignant etiology (10).
The global prevalence of peptic ulcer disease has reduced over the past years, and these changes occur due to significant changes in the risk factors involving the reduction of H. pylori infection and the widespread use of NSAID drugs and aging of the patients (11). Peptic ulcers affect approximately four million people annually. Duodenal ulcers often appear between ages 30 and 50 years and are more common in men, but stomach ulcers occur after the age of 60 years (11). The prevalence of ulcers over the last decades has decreased. Generally, duodenal ulcers are more common than gastric ulcers (12).
2. Objectives
This cross-sectional study was conducted in Duhok, Kurdistan, Iraq, reporting the prevalence and risk factors associated with peptic ulcers among patients undergoing upper GIT endoscopy.
3. Methods
3.1. Design of Study and Measurement
This cross-sectional study was conducted in Duhok, Kurdistan, Iraq, between August 11 and December 17, 2022. A total of 218 patients (116 males and 102 females) who underwent upper gastrointestinal endoscopy by specialists at Azadi Teaching Hospital were interviewed face-to-face. The participants ranged from 18 to 81 years, with a mean age of 39 ± 12.9. The procedure involved using a flexible endoscope called PENTAXI-scan 5000, inserted through the mouth down to the esophagus, stomach, and duodenum. A gel containing 2% lidocaine HCL was applied before inserting the endoscope tube. In some cases, intravenous sedation (midazolam injection) was administered to alleviate anxiety and discomfort associated with the procedure. Patients fasted for 8 hours before the procedure, typically lasting 5 - 15 minutes.
The study questionnaire consisted of three parts:
(1) Socio-demographic characteristics, including age, sex, educational level, marital status, and occupational status.
(2) Questions about major symptoms of peptic ulcers, including abdominal pain, vomiting, heartburn, and regurgitation.
(3) Questions about dietary habits such as smoking, coffee drinking, NSAID use, spicy food, and alcohol consumption.
3.2. Inclusion/Exclusion Criteria
The inclusion criteria were to be over 18 years of age, residing in the Kurdistan region of Iraq, and willing to participate. Conversely, individuals under 18 or over 85, those using proton pump inhibitors (PPI), and those with incomplete or missing information on the questionnaires were excluded.
3.3. Rapid Urease Test
A biopsy was taken and immersed in rapid urease test slides during an upper gastrointestinal endoscopy. When H. pylori is present in the biopsy sample, the urease enzyme the bacteria produces breaks down urea in the test solution. This process increases pH, causing the color to change to pink or red, indicating a positive result for H. pylori.
3.4. Ethical Approval
The study protocol, design, procedure, and format of informed consent were approved by the ethics and scientific committee of the College of Medicine, University of Zakho, Kurdistan region of Iraq. Informed consent was obtained from all participants before starting sampling.
3.5. Statistical Analysis
All the data were analyzed using GraphPad Prism version 9.3.1 software. Descriptive statistics, including frequencies and percentages, were employed to examine respondents' characteristics and responses. The chi-Square test explored the association between peptic ulcers and associated factors. A P-value < 0.05 was considered statistically significant
4. Results
The prevalence of peptic ulcers among the participants was 30 (13.8%), including duodenal ulcers (12.4%) and gastric ulcers (1.4%) (Table 1).
| Peptic Ulcer | Frequency (%) |
|---|---|
| Yes (30, 13.8%) | |
| Gastric ulcer | 3 (1.4) |
| Duodenal ulcer | 27 (12.4) |
| No | 188 (86.2) |
| Total | 218 (100) |
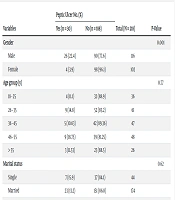
The relationship between peptic ulcer and socio-demographic characteristics and dietary habits is presented in Table 2. There was only a significant association between H. pylori (P = 0.001) and gender (P = 0.001) with peptic ulcer. However, no significant associations were found between peptic ulcer and other dietary habits (Table 2).
| Variables | Peptic Ulcer No. (%) | Total (N = 218) | P-Value | |
|---|---|---|---|---|
| Yes (n = 30) | No (n = 188) | |||
| Gender | 0.001 | |||
| Male | 26 (22.4) | 90 (77.6) | 116 | |
| Female | 4 (3.9) | 98 (96.1) | 102 | |
| Age group (y) | 0.77 | |||
| 18 - 25 | 4 (11.1) | 32 (88.9) | 36 | |
| 26 - 35 | 9 (14.8) | 52 (85.2) | 61 | |
| 36 - 45 | 5 (10.63) | 42 (89.36) | 47 | |
| 46 - 55 | 9 (18.75) | 39 (81.25) | 48 | |
| > 55 | 3 (11.53) | 23 (88.5) | 26 | |
| Marital status | 0.62 | |||
| Single | 7 (15.9) | 37 (84.1) | 44 | |
| Married | 23 (13.2) | 151 (86.8) | 174 | |
| Residence | 0.42 | |||
| Urban | 27 (14.8) | 156 (85.2) | 183 | |
| Rural | 3 (9) | 32 (91.4) | 35 | |
| Level of education | 0.65 | |||
| Uneducated | 1 (9) | 10 (90.9) | 11 | |
| Primary school | 4 (10) | 36 (90) | 40 | |
| Middle school | 7 (16.7) | 35 (83.3) | 42 | |
| High school | 11 (17.5) | 52 (82.5) | 63 | |
| University | 6 (10.7) | 53 (89.8) | 59 | |
| Post graduated | 1 (33.3) | 2 (66.6) | 3 | |
| Smoking | 0.14 | |||
| Yes | 3 (6.7) | 42 (93.3) | 45 | |
| No | 27 (15.6) | 146 (84.4) | 173 | |
| NSAID use | 0.36 | |||
| Yes | 10 (17.9) | 46 (82.1) | 56 | |
| No | 20 (12.3) | 142 (87.7) | 162 | |
| Iron use | 0.37 | |||
| Yes | 5 (19.2) | 21 (80.8) | 26 | |
| No | 25 (13) | 167 (87) | 192 | |
| Corticosteroid use | 0.71 | |||
| Yes | 1 (6.7) | 14 (93.3) | 15 | |
| No | 29 (14.3) | 174 (85.7) | 203 | |
| Previous peptic ulcer | 0.99 | |||
| Yes | 2 (13.3) | 13 (86.7) | 15 | |
| No | 28 (13.8) | 175 (86.2) | 203 | |
| Family history | 0.61 | |||
| Yes | 6 (16.2) | 31 (83.8) | 37 | |
| No | 24 (13.3) | 157 (86.7) | 181 | |
| H. pylori | 0.001 | |||
| Yes | 15 (55.6) | 12 (44.4) | 27 | |
| No | 15 (7.9) | 176 (92.1) | 191 | |
| Body Mass Index | 0.32 | |||
| < 25 | 17 (11.6) | 129 (88.3) | 146 | |
| 25 - 30 | 10 (16.7) | 50 (83.3) | 60 | |
| > 30 | 3 (25) | 9 (75) | 12 | |
| Coffee consumption | 0.21 | |||
| Yes | 1 (4.2) | 23 (95.8) | 24 | |
| No | 29 (14.9) | 165 (85.1) | 194 | |
| Spicy food | 0.14 | |||
| Yes | 3 (6.4) | 44 (93.6) | 47 | |
| No | 27 (15.8) | 144 (84.2) | 171 | |
Table 3 presents the characteristics of peptic ulcers among the studied cases. The primary complaint was abdominal pain (39%), followed by heartburn (28.4%), vomiting (6.9%), and loss of appetite (0.5%). Additionally, Table 3 displays the complications of peptic ulcers among the studied cases, with 5% reporting melena, while only 2.3% reported hematemesis.
| Characteristics | Frequency (%) |
|---|---|
| Signs and symptoms | |
| Abdominal pain | 85 (39) |
| Heartburn | 62 (28.4) |
| Vomiting | 15 (6.9) |
| Regurgitation | 2 (0.9) |
| Loss of apatite | 1 (0.5) |
| Abdominal pain and heartburn | 34 (15.6) |
| Abdominal pain and vomiting | 12 (5.5) |
| Abdominal pain and regurgitation | 7 (3.2) |
| Complications | |
| Hematemesis | 5 (2.3) |
| Melena | 11 (5) |
| Hematemesis and melena | 1 (0.5) |
| No | 201 (92.2) |
5. Discussion
This cross-sectional study involved 218 participants aged between 18 and 81 years. The study aimed to measure the prevalence rate and risk factors of peptic ulcers and to determine the main etiologies behind this disease among patients undergoing upper GIT endoscopy. The prevalence of peptic ulcers was 13.8%, with rates of 1.4% and 12.4% observed for gastric and duodenal ulcers, respectively. The findings were lower than those of a study in Saudi Arabia, which reported a prevalence rate of 21.9% (gastric ulcer: 16.2%; duodenal ulcer: 6.2%) (13). Additionally, the present research results were lower than those of another study conducted in Basrah, Iraq (14), which reported a prevalence rate of 51.7% for patients with peptic ulcers. However, the findings were higher than those of European endoscopic epidemiologic studies, which reported prevalence rates ranging from 4.1% (gastric ulcer: 2.0%; duodenal ulcer: 2.1%) (15) to 6.2% (gastric ulcer: 2.3%; duodenal ulcer: 3.9%) (16). Another study conducted in Iran reported that the prevalence of peptic ulcers was 8.20%, with rates of 3.26% and 4.94% for gastric and duodenal ulcers, respectively (17). The discrepancy between the prevalence of peptic ulcers in this study and findings from other research could be attributed to several factors, including study population, geographical variation, study design and study period, sample size, and diagnostic techniques.
A higher prevalence of peptic ulcer disease occurred among men in the present study, which agrees with the study conducted in Basrah, Iraq (14). The findings indicated that NSAID use has a non-significant risk for peptic ulcer disease (PUD), which contrasts with the studies conducted in Basrah, Iraq, and elsewhere that indicate a strong association between NSAID and PUD (14, 17).
A study in Zambia reported that among PUD cases, 40% confirmed the use of NSAID, 34% were cigarette smokers, and 57% were regular alcohol drinkers (18). In the Iranian study (17)), results showed that H. pylori infection (2.1%), smoking (1.1%), and NSAID use (1.3%) were the main risk factors for gastric ulcers. In a meta-analysis investigating the risk factors for peptic ulcer disease, it was shown that cigarette smoking H. pylori infection is the main risk factors (19). A study in the KSA reported that the prevalence of H. pylori infection among patients with peptic ulcers was 63% (20). In addition, a study conducted in Singapore showed that H. pylori prevalence was 67.9% in gastric ulcers, 85.1% in duodenal ulcers, and 85.7% in combined gastric and duodenal ulcers (21). This study found a significant association between H. pylori infection and PUD. H. pylori infection is very common in Iraq (4, 22). Previous studies have shown that the prevalence of infection was around 80% (23). Studying the H. pylori antibiotic sensitivity pattern in our country showed a high resistance rate to commonly used antibiotics for the eradication of the microorganism (24-26). This increases the challenge and the burden of dealing with such an infection.
In this study, the percentage of alcoholic patients was very small, resulting in a non-significant value. Corticosteroid use also showed a non-significant risk of developing PUD, which aligns with a study conducted in Basrah (14). However, another study at Konkuk University Medical Center in Korea demonstrated a strong association between alcohol drinking and PUD (27). In this study, oral iron supplementation had a non-significant effect on developing peptic ulcers. This contrasts with a study conducted in Basrah, which indicated a significant risk for PUD development (14). This study's most common complication of peptic ulcers was melena (5%), followed by hematemesis (2.3%). A Saudi study on adolescents and children found that hematemesis and melena occurred in (13%) and (8%), respectively (28).
5.1. Conclusions
In conclusion, this study revealed that approximately 13.8% of individuals undergoing upper gastrointestinal endoscopy exhibit peptic ulcers, with 12.4% manifesting duodenal ulcers and 1.4% exhibiting gastric ulcers. Sex and H. pylori infection emerged as identified risk factors for peptic ulcer disease. The variability in associated risk factors with PUD may be attributed to the multifactorial nature of the inflammatory and ulcerative processes, influenced by genetic predisposition and environmental conditions. Further research is imperative to comprehensively investigate the impact of diverse factors on the development of PUD.