1. Background
Urolithiasis, as one of the most prevalent uronephrologic disorders (1), can be affected by genetic polymorphisms, metabolic defects, and environmental factors (2). The prevalence of urolithiasis is 4 - 20% in developed countries, and the incidence of this disorder is increasing (3). Calcium is the main crystal-line constituent in most kidney stones, up to 80%, and excessive calcium absorption increases the tendency of stone formation (2).
Vitamin D, as a hormone, has an essential role in the metabolism of calcium and phosphate. The active form of vitamin D, 1,25 (OH)2-D3, binds to the nuclear vitamin D receptor (VDR) and exerts its function on gene transcription in target tissues (4). Maternal status of vitamin D and prenatal vitamin D deficiency may influence kidney stone formation (5). The high prevalence of inadequate vitamin D has been reported in individuals with urolithiasis. VDR, which is necessary for the function of vitamins, has been increased by 2-fold in males with kidney stones. The VDR gene variants, through affecting the level of VDR protein, might be involved in the pathogenesis of urolithiasis. The role of genes and genetic background has been suggested for susceptibility to urolithiasis. The study of family history indicated an increased risk of disease by 2.57 times in males (1).
There are some single nucleotide polymorphisms in the VDR gene, including the BsmI (A > G, rs1544410) on the last intron and TaqI (T > C, rs731236) on the 9th exon of the 3ʾ terminal (6). The VDR gene polymorphisms of TaqI and BsmI do not alter the structure of VDR and affect the mRNA stability or alteration of vitamin D activity through translation regulation (3). Polymorphisms in VDR genes have been implicated in the efficiency of VDR translation and the stability of its mRNA, which can affect VDR protein expression (5). The VDR gene polymorphisms (ApaI, BsmI, TaqI, and FokI) could affect the VDR protein activity and expression and vitamin D signaling pathway so that they might play a significant role in kidney stone formation (5).
There are some meta-analyses and reports related to the influence of VDR polymorphisms on the risk of urolithiasis, but these have had controversial results (1-3, 5, 7-10). In most of the meta-analyses, due to insufficient data or deviation from the Hardy-Weinberg equilibrium, the association between the VDR polymorphisms and urolithiasis in children has not been examined separately (1, 3, 7).
2. Objectives
Since some polymorphisms in the VDR gene might affect the risk of kidney stone formation and urolithiasis, and the presence of few studies with inconsistent results, the present study aimed to find the possible association of the polymorphisms of TaqI and BsmI of the VDR gene in relation to the serum levels of calcium and vitamin D influence with the risk of urolithiasis among children from Kermanshah province, Western Iran. Since the polymorphisms in the genes, including VDR, could be ethnically dependent, this study is the first study among children with Kurdish ethnic background to detect the effects of VDR TaqI and BsmI variants and haplotypes on urolithiasis regarding the serum calcium and vitamin D levels.
3. Methods
In this case-control study, 68 children with urolithiasis and 67 healthy controls were studied. Cases were selected from children with urolithiasis who were referred to the pediatric nephrology clinic of Imam Reza Hospital of Kermanshah University of Medical Sciences in 2020. Inclusion criteria were the age below 18 years, having a kidney stone, and the size of the stone equal to or more than 3 millimeters. Exclusion criteria were intake of steroids, using a therapeutic dose of vitamin D, intake of drugs affecting electrolyte balance, renal failure, the presence of recurrent urinary tract infection, the presence of hypercalcemia and hyperphosphatemia, hypervitaminosis D, hyperparathyroidism, genetic disorders that increase susceptibility to kidney stones. For each patient, a control was selected from children who were referred to this center for their annual evaluations without any history of kidney stones. The mean age of patients was 2.9 years, and the mean age of controls was 5.2 years. Patients and controls were sex-matched, with 35 males and 33 females in patients and 36 males and 31 females in controls.
3.1. Vitamin D
The vitamin D status was determined by measuring the serum level of 25 (OH)-D3 using the Immunodiagnostic Systems Limited (IDS) EIA kit. An adequate vitamin D level was defined as 25 (OH)-D levels above 50 nmol/L (20 ng/mL). Levels between 30 and 50 nmol/L (12 - 20 ng/mL) were classified as insufficient, and levels below 30 nmol/L (12 ng/mL) were considered deficient (6). The serum calcium level was measured using the Arsenazo III reagent and a colorimetric method.
3.2. Genotyping
DNA was extracted from EDTA-treated whole blood using the phenol-chloroform method (11). The integrity of the DNA was assessed using 1% agarose gel electrophoresis. The concentration and purity of the extracted DNA were measured using a Thermo Nanodrop spectrophotometer by determining the absorbance at 260 nm and calculating the ratio of absorbance at 260 nm to 280 nm, respectively.
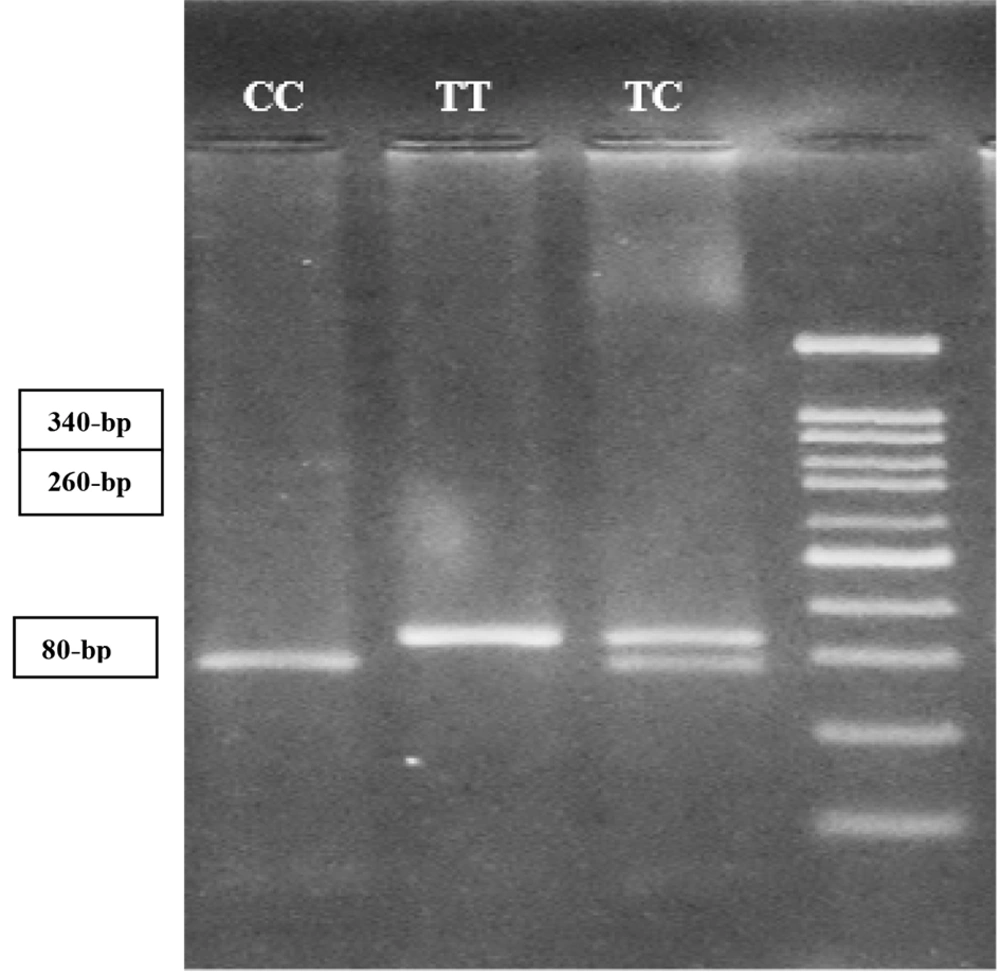
Detection of TaqI T > C (rs731236) polymorphism in exon 9 of the VDR gene was performed by the forward primer of 5´-CAG AGC ATG GAC AGG GAG CAA G-3´ and the reverse primer of 5´-GGA TGT ACG TCT GCA GTG TG-3´ using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method (6). PCR amplification was conducted in a total volume of 25 μL containing 500 ng of genomic DNA as template, 2.5 μL of 10x PCR reaction buffer, 1.5 mM MgCl2, 0.2 mM of dNTPs, 0.5 μM of each forward and reverse primers, and 1 unit of Taq DNA polymerase. Amplification of a fragment with 340-bp from VDR TaqI was carried out for 35 cycles at 95°C for 45 seconds, 66°C for 1 min, and 72°C for 1 min, with a final extension period of 5min at 72°C. After amplification, 10 μL of PCR products were subjected to overnight digestion with three units of TaqI at 37°C. Digested PCR products were detected on a 2.5% agarose gel stained with DNA Safe Stain under ultraviolet light. The PCR product with 340-bp fragment remained intact in the presence of the TT genotype. The PCR product of the 340-bp fragment was digested into two fragments of 260- and 80-bp while in the presence of the CC mutant genotype. Three fragments, 340-, 260-, and 80-bp, were produced in the presence of a heterozygous genotype of TC (Figure 1).
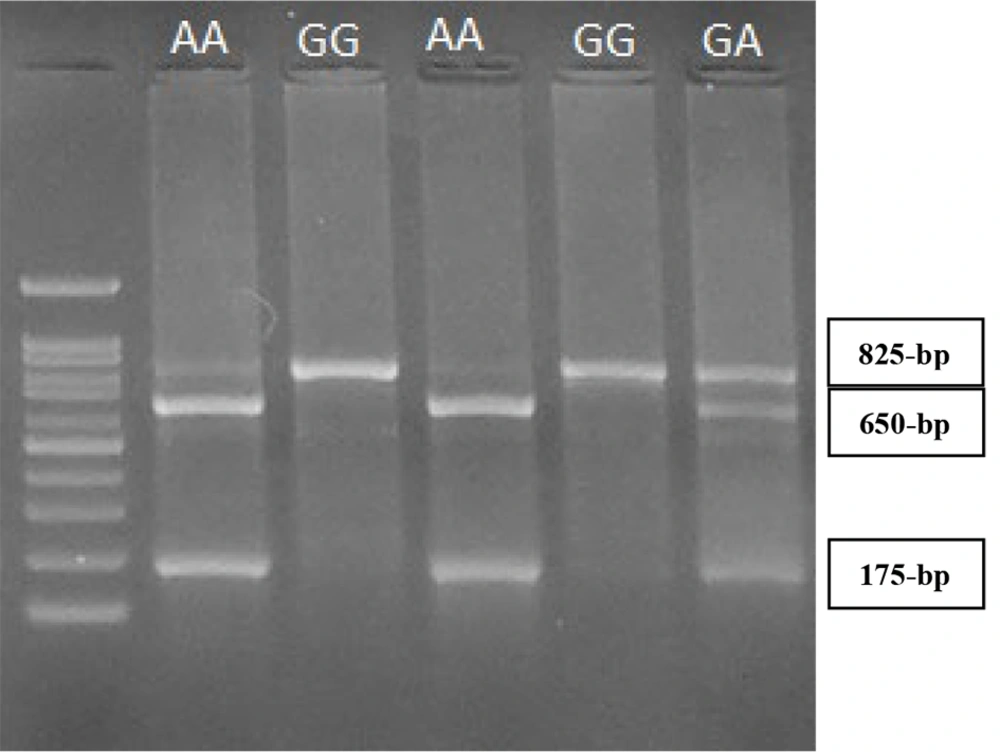
The BsmI G > A (rs1544410) polymorphism in intron 8 of the VDR gene was identified by PCR-RFLP method using the forward primer of 5´-CAA CCA AGA CTA CAA GTA CCG CGT CAT GA-3´ and the reverse primer of 5´-AAC CAG CGG GAA GAG GTC AAG GG-3´ (6). Briefly, a fragment with 825-bp was amplified using 35 cycles at 95°C for 45 seconds, 66°C for 1 min, and 72°C for 1min, with a final extension period of 5min at 72°C. After amplification, 10μl of PCR products were subjected to overnight digestion with two units of BsmI at 37°C. Digested PCR products were detected on a 2.5% agarose gel stained with DNA Safe Stain under ultraviolet light. The 825-bp fragment is not digested by the restriction enzyme of BsmI in the presence of the GG genotype. However, two fragments with 650- and 175-bp are produced in the presence of the AA genotype. The presence of GA genotypes results in three fragments with 825-, 650-, and 175-bp (Figure 2).
Informed written consent was obtained from parents of children before participation in the study. The study was approved by the Ethics Committee of Kermanshah University of Medical Sciences and was in accordance with the principles of the Declaration of Helsinki II.
3.3. Statistical Analysis
The significance of differences in the genotype and the allele frequencies of TaqI and BsmI polymorphisms between patients and contro
ls was calculated using the χ2 test. A two-tailed student’s t-test was used to compare quantitative data. The SPSS (SPSS Inc., Chicago, IL, USA) statistical software package version 16.0 was used for statistical analysis.
4. Results
Table 1 demonstrates the characteristics of children with urolithiasis and controls. The mean levels of 25 (OH)-D3 were 32.8 ± 20.2 ng/mL in patients and 31.5 ± 19.3 ng/mL in controls (P = 0.68). The serum calcium level was 9.9 ± 1.7 mg/dL in patients compared to 9.97 ± 0.5 mg/dL in controls (P = 0.74)
| Variables | Patients (n = 68) | Controls (n = 67) | P-Value |
|---|---|---|---|
| Age (y) | 2.9 ± 3.1 | 5.2 ± 3.9 | < 0.001 |
| Gender | |||
| Male | 35 (51.5) | 36 (53.7) | |
| Female | 33 (48.5) | 31 (46.3) | |
| 25 (OH)-D (ng/mL) | 32.8 ± 20.2 | 31.5 ± 19.3 | 0.68 |
| Calcium (mg/dL) | 9.9 ± 1.7 | 9.97 ± 0.5 | 0.74 |
a Values are expressed as No. (%) or Mean ± SD.
4.1. TaqI T > C Genotypes
Distribution of TaqI T > C genotypes was in Hardy-Weinberg equilibrium in children with urolithiasis (χ2 = 1.31, P > 0.1) and controls (χ2 = 0.38, P > 0.1). The frequency of the TaqI C allele was 36% in patients compared to 32.1% in controls (P = 0.49) (Table 2). Classification of patients in three age groups of 0 - 5 years, >5 to 10 years, and more than ten years revealed a significantly higher frequency of the CC genotype in the age group >5 to 10 years compared to the age groups of 0 - 5 years and >10 years (Table 3).
| Parameters | Patients (n = 68) | Controls (n = 67) | Overall χ2 | P-Value |
|---|---|---|---|---|
| TaqI genotypes | 0.76 | |||
| TT | 30 (44.1) | 32 (47.8) | 0.53 | |
| TC | 27 (39.7) | 27 (40.3) | ||
| CC | 11 (16.2) | 8 (11.9) | ||
| Alleles | 0.49 | |||
| T | 87 (64) | 91 (67.9) | ||
| C | 49 (36) | 43 (32.1) | 0.46 |
a Values are expressed as No. (%).
| Parameters | 0 - 5 Years | > 5 to 10 Years | > 10 Years |
|---|---|---|---|
| TaqI genotypes | |||
| TT | 23 (41.1) | 4 (57.1) | 2 (66.7) |
| TC | 25 (44.6) | 0 (0) | 1 (33.3) |
| CC | 8 (14.3) | 3 (42.9) | 0 (0) |
a χ2 = 7.4, P = 0.006, Compared to the TC genotype in the age group of 0 - 5 years.
b χ2 = 4, P = 0.046 χ2 compared to the TC genotype in the age group > 10 years.
4.2. BsmI G > A Genotypes
The distribution of BsmI genotypes was in Hardy-Weinberg equilibrium in patients (χ2 = 0.0, P > 0.1) and also in controls (χ2 = 0.32, P > 0.1). Table 4 demonstrates the distribution of BsmI genotypes and alleles in patients and controls. No significant difference was detected in the frequency of A allele between patients (41.9%) and controls (42.5%, P = 0.91). However, a significantly lower frequency of BsmI AA genotype (11.3%) was found among patients with Ca levels of ≤ 10.8 mg/dL compared to those patients with Ca levels of > 10.8 mg/dL (80%, P = 0.002) as indicated in Table 5.
| Parameters | Patients (n = 68) | Controls (n = 67) | Overall χ2 | P-Value |
|---|---|---|---|---|
| BsmI genotypes | 0.18 | 0.91 | ||
| GG | 23 (33.8) | 21 (31.3) | ||
| GA | 33 (48.5) | 35 (52.2) | ||
| AA | 12 (17.6) | 11 (16.4) | ||
| Alleles | 0.011 | 0.91 | ||
| G | 79 (58.1) | 77 (57.5) | ||
| A | 57 (41.9) | 57 (42.5) |
a Values are expressed as No. (%).
a Values are presented as No. (%).
b Compared to GA genotype.
c compared to GG genotype.
Haplotype analysis of VDR polymorphisms is depicted in Table 6. As shown in Table 6, compared to the reference haplotype of TG (Taq I T, BsmI G), the frequency of other haplotypes was not significantly different when comparing patients and controls.
| TaqI | BsmI | Haplotype Frequency | χ2 | P | |
|---|---|---|---|---|---|
| Patients, N = 136 | Controls, N = 134 | ||||
| T | G | 55 (40.4) | 60 (44.8) | 1 | |
| C | G | 24 (17.6) | 17 (12.7) | 1.38 | 0.23 |
| T | A | 32 (23.5) | 29 (21.6) | 0.34 | 0.55 |
| C | A | 25 (18.4) | 28 (20.9) | 0.006 | 0.93 |
5. Discussion
The active form of vitamin D is 1,25(OH)2-D3. However, its precursor of 25 (OH)-D is evaluated for vitamin D status (4). In the present study, the 25 (OH)-D3 level was not significantly different comparing children with urolithiasis and controls. Genetic background could play an essential role in the variation of the circulating levels of 25 (OH)-D among individuals. The active form of vitamin D binds to the VDR and affects different genes and metabolic pathways (4).
This study indicated the absence of an association between the VDR TaqI and BsmI polymorphisms and the risk of urolithiasis among children from Western Iran. In addition, haplotype analysis stated the lack of association between VDR haplotypes and the risk of urolithiasis. Studying patients in three age groups indicated a significantly higher frequency of the TaqI CC genotype in the age group > 5 to 10 years compared to other age groups (0 - 5 years and > 10 years). A significantly lower frequency of BsmI AA genotype was detected among patients with Ca levels of ≤ 10.8 mg/dL compared to those patients with Ca levels of > 10.8 mg/dL. The role of VDR in calcium metabolism has been suggested by biological evidence. In the genetic hypercalciuric rat model, increased intestinal numbers of VDR enhanced calcium absorption (2). An association between TaqI polymorphism and the risk of urolithiasis was reported in a recent study in Kerman among 90 pediatric urolithiasis patients and 90 healthy children (12). This study demonstrated that the C allele and the CC genotype of TaqI polymorphism were considerably linked with a higher risk of pediatric urolithiasis (12). Although this study showed a higher frequency of the TaqI C allele and the TaqI CC genotype among patients than in the controls, it did not reach statistical significance. Differences in the results obtained between our study and the study by Parvaresh et al. (12) could be attributed to the difference in the sample size and different ethnic backgrounds of the studied individuals.
There is some meta-analysis examining the association between the VDR gene polymorphisms and the risk of urolithiasis but with various results. In a meta-analysis by Lin et al., including 17 studies, no significant association was found between ApaI and BsmI polymorphisms and the risk of urolithiasis. However, the FokI f and the TaqI t alleles increased the urolithiasis risk in the subgroup of Asians (2). In another meta-analysis consisting of 23 case-control studies from Asians and Caucasians, the VDR gene polymorphisms of the ApaI and TaqI were associated with urolithiasis (1), indicating the role of ethnicity in susceptibility to urolithiasis. In another meta-analysis by Liu et al. (7) analyzing 20 case-control studies, only TaqI polymorphism was associated with the risk of urolithiasis. In contrast, the FokI, BsmI, and ApaI did not affect the risk of this disorder. Imani et al. conducted a meta-analysis of 33 studies to examine the association of four VDR polymorphisms with urolithiasis, including FokI (rs2228570), ApaI (rs7975232), TaqI (rs731236), and BsmI (rs1544410), and found that ApaI and TaqI polymorphisms are associated with increased risk of urolithiasis among East Asian and Caucasian populations (3). However, in another meta-analysis by Amar et al. in the same year, among four polymorphisms of FokI, ApaI, TaqI, and BsmI, only the VDR FokI polymorphism was associated with the risk of urolithiasis, especially in Asians (8). In another recent systematic review and meta-analysis of 14 studies among Asians, the FokI polymorphism of the VDR gene in a recessive model was associated with recurrent kidney stone risk. However, the VDR TaqI heterozygous genotype had a protective role against recurrent kidney stone risk (5). In addition, Gonzalez-Castro et al. (13), in a meta-analysis, reported that the TaqI polymorphism was associated with a decreased risk of nephrolithiasis in the heterozygous model and the BsmI polymorphism had a protective association against nephrolithiasis. In a study among Turkish children with kidney stones, the VDR gene polymorphisms were not the risk factors for urolithiasis (9). Moreover, in another study from Turkey, the BsmI and TaqI genotype distribution in stone-forming patients was similar to those in controls (14). In another study among infants (mean age around seven months) from Turkey, the BsmI and the TaqI polymorphisms were suggested as genetic markers for infantile urolithiasis (10). Among a population from Pakistan, no significant association was detected between six studied VDR polymorphisms and the risk of urolithiasis (8). In a recent systematic review and meta-analysis, Mohammadi et al. (15) suggested the multifactorial nature of the stone formation and emphasized the role of environmental factors could explain contradictory results in the literature.
The presence of various findings might be due to differences in the ethnicity and geographic diversity of the serum level of vitamin D and also the VDR gene expression. Environmental factors affect the risk of disease as seasonal differences influence the serum level of vitamin D (3). Lifestyle, age, and gender need to be considered in the evaluation of VDR gene polymorphisms with urolithiasis susceptibility (3). Metabolic abnormalities are more associated with urolithiasis in children compared to adults (10).
5.1. Conclusion
In the present study, the VDR TaqI and BsmI polymorphisms were not associated with the risk of urolithiasis among children from Western Iran. However, a higher frequency of the TaqI CC genotype in the age group > 5 to 10 years was found. In addition, a significantly lower frequency of the BsmI AA genotype was found in patients with Ca levels of ≤ 10.8 mg/dL than in patients with Ca levels of > 10.8 mg/dL. VDR gene polymorphism may affect the number of VDR in the intestine and influence calcium metabolism.