1. Introduction
Rendu-Osler-Weber syndrome, also known as hereditary hemorrhagic telangiectasia (HHT), is a fibrovascular autosomal dominant disorder with an overall incidence of 1 - 20 cases per 100000 in the general population (1, 2). However, there is no report of its prevalence rate in Iran, and we found only a report from the Kerman province of Iran in 2007, which presented three cases of HTT in a family (3). They made an initial diagnosis of HHT in a 63-year-old man based on clinical symptoms such as anemia, history of recurrent epistaxis, hematemesis, melena, and multiple telangiectasia in the gastrointestinal tract (3). Clinical presentations of HTT mainly include recurrent epistaxis, mucocutaneous telangiectasia, visceral arteriovenous malformations, and also a positive family history (4, 5). Diagnosis of HTT is approved upon presentation of at least three of these four criteria (4, 5). Visceral arteriovenous malformations (AVMs) frequently affect the pulmonary, hepatic, and cerebral circulation, and recurrent epistaxis is the most frequent manifestation, which usually occurs since childhood (6). It has been suggested upregulation of VEGF, TGF-β1, and ALK1 may play a key role in HHT, as a multi-systemic angiogenic disorder, by uncontrolled angiogenesis following the formation of disorganized vessels prone to bleeding (7-10). Thus, bevacizumab, a humanized anti-VEGF antibody, can be considered a safe therapeutic option to control symptoms of patients with HHT by VEGF inhibition (11). Indeed, some reports have shown that intravenous bevacizumab therapy is associated with the reduction of epistaxis episodes, telangiectasias, bleedings, and also blood transfusions (12). Other treatment options include nasal packing, vessel ligation, dermo septoplasty, hormonal therapy, laser treatment, and imaging using Doppler US, CT, and MRI are often applied to detect vascular lesions in patients (13).
2. Case Presentation
A 77-year-old female patient came to the internal medicine ward of Imam-Khomeini Hospital in Kermanshah, Iran, complaining of epistaxis with a pale appearance and hemoglobin (Hb): 5.9 g/dL. For about 40 years, she had repetitive epistaxis and had been frequently hospitalized to inject blood transfusions due to her low hemoglobin level. Besides, the first hemoglobin in the 15 hospital admissions was recorded from 2015 to 2021 (Table 1).
| Date | 2015, Aug | 2016, Sep | 2017, Aug | 2017, Nov | 2018, Jul | 2018, Sep | 2018, Dec | 2019, May | 2019, Feb | 2020, Apr | 2020, May | 2020, Jul | 2020, Sep | 2021, Jul | 2021, Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hb (g/dL) | 4.4 | 6.4 | 3.4 | 2.2 | 3.3 | 2.7 | 2.5 | 3.1 | 3.8 | 2.3 | 3.3 | 3.1 | 3.3 | 1.6 | 2.1 |
Abbreviation: Hb, hemoglobin.
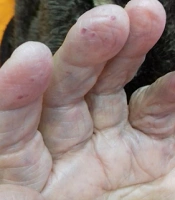
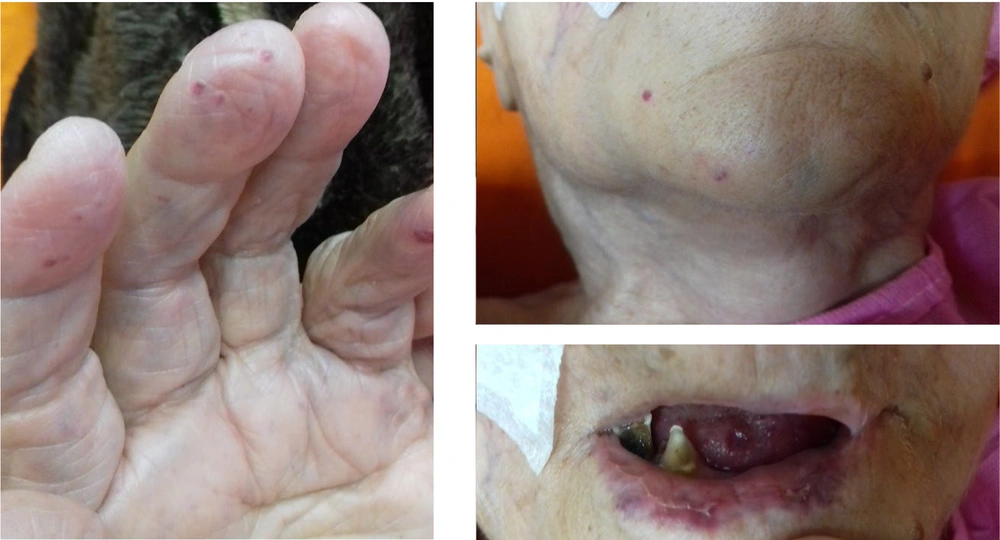
In addition, her daughter and one of her grandsons with recurrent epistaxis were diagnosed with HHT. Furthermore, during physical examination, telangiectasia was seen on the tip of her hand, fingers, face, and lips (Figure 1). So far, the patient had no history of hematemesis, melena, chest pain, dyspnea, or vertigo on admission. She was hypertensive and received losartan 25mg daily. An upper endoscopy was performed to assess gastrointestinal telangiectasia following her previous hospital admission for melena and hematemesis following massive epistaxis. Owing to normal endoscopy, color Doppler sonography of her abdominal veins was conducted due to ascites and bilateral pleural effusion post massive blood transfusion. A dilated portal vein (15 mm diameter) was shown with a tortuous appearance and cavernous changes in the hilum of the liver. Furthermore, dilation of the right hepatic vein (10 mm diameter) was reported due to congestive heart failure. However, her echocardiography had normal left ventricular ejection fraction (55%) with moderate tricuspid regurgitation and severe mitral regurgitation. Overall, our patient fulfills all four criteria of HHT, including epistaxis, telangiectasia, visceral AVMs, and family history of HTT.
3. Discussion
Nasal packing was performed to prevent further bleeding, and the patient was given four units of blood transfusions during hospitalization. Tranexamic acid was prescribed for years without any benefit to this patient. The last laboratory tests at discharge showed Hb: 10.3 g/dL, Hct: 36.5%. In addition, the patient was intravenously injected with the first dose (5 mg/kg) of Avastin, and no adverse effects like hypertension crisis appeared during its infusion. Bevacizumab, an anti-angiogenic antibody, is a potential VEGF-targeted treatment in HHT. A HIBIT-Bleed study reported that the number of transfused red blood cell units decreased by 82% compared to 6 months before bevacizumab treatment. However, adverse events such as hypertension (18%), fatigue, and proteinuria were commonly observed in patients (14). Furthermore, the efficacy and safety of bevacizumab in patients with HHT have been evaluated in a multicenter randomized trial that included 24 patients in two groups. Six intravenous injections of bevacizumab (5 mg/kg dose every two weeks) were associated with a significant decrease (at least 50%) of blood transfusions in 63.6% of patients versus 33.3% in the placebo group. Moreover, hemoglobin levels increased at six months compared to the placebo group (P = 0.02). The adverse effects related to treatment were tolerable and well-managed. Systemic hypertension was observed in 8% of patients (n = 2), which was easily controlled, and other side effects such as asthenia (n = 5), infections (n = 6), and headaches (n = 3) were found without bleeding and clotting events (15). Dupuis-Girod et al. showed that six cycles of bevacizumab therapy in 25 patients with HTT could improve the number, duration, and severity of epistaxis as well as the level of hemoglobin. However, adverse effects, including grade 3 hypertension (n = 2), headache, nausea, abdominal pain, diarrhea, rash, and muscle pain, were reported and successfully managed (16). Furthermore, a meta-analysis study (including seven documents) focused on bevacizumab's effects on HTT-associated epistaxis among 359 patients. This study indicated that bevacizumab treatment reduces epistaxis severity (P = 0.01) but does not significantly impact the duration or number of epistaxis patients with HHT versus those in the control group. In addition, each group's adverse effects rate was similar, and there was no significant difference (17). Many studies have indicated the clinical efficacy of bevacizumab therapy in improving epistaxes, hemoglobin level, and blood transfusion requirements in patients with HHT concurrent to low and manageable adverse effects.
The patient and her family refused to continue Avastin, and at the last admission, she was hospitalized to receive packed cells only to correct anemia for cataract surgery. She was referred to an otolaryngologist for intra-nasal injection of bevacizumab based on some successful reports which showed efficacy of bevacizumab without serious adverse events (18).
Herein, we present the second family of HHT during the last 15 years in Iran. She has cutaneous telangiectasias, hepatic AVMs, and a positive family history. She may stop the treatment course because there is not enough evidence for the safety and efficacy of bevacizumab, especially in this hypertensive patient, its cost, and the patient's and her family's preferences. We hope the intra-nasal injection of Avastin will be effective if she follows the otolaryngologist's recommendation.