1. Background
The reasons behind the range of COVID-19 disease severity and the development and characteristics of protective immunity against COVID-19 are still not fully understood. Numerous studies have examined immune responses to COVID-19 in different groups, even in individuals without confirmed infection. The immune system plays a crucial role in suppressing viral replication and managing infections, such as COVID-19 (1). It is crucial to acknowledge that an excessively active or imbalanced immune response can result in the triggering of severe inflammation during viral infections (2). In the case of COVID-19, the immune system recognizes the presence of the SARS-CoV-2 virus and initiates an immune response to eliminate the virus (3). This response involves the activation of adaptive and innate immune cells, and the release of cytokines and chemokines (4). These immune components work together to combat the virus and clear the infection (5, 6). The excessive immune responses to SARS-CoV-2 can be associated with increased inflammation and increased chances of morbidity and mortality (7). The roles played by several molecules in activation or inhibition of the immune cell functions to SARS-CoV-2 have been demonstrated previously (8). The roles of various molecules in the pathogenesis of severe COVID-19 in different ethnic groups still require further clarification. Suppressor of cytokine signaling 1 (SOCS1) is a main cytokine signaling pathway inhibitor (9). Accordingly, the molecule can suppress the cytokine-dependent immune cell, inducing T and B lymphocytes (9). Due to the roles played by the molecule to regulate the immune responses, it has been hypothesized that it might be suppressed by some unknown mechanisms during severe COVID-19. Additionally, interleukin-29 (IL-29) and lysosomal trafficking regulator (LYST) are two key molecules that induce anti-viral properties in immune and non-immune cells (10, 11). For example, it has been documented that IL-29 plays key roles in induction of cellular immunity (12). Also, LYST is a main immune cell-related molecule that its dysfunction can lead to Chediak-Higashi syndrome, an immunodeficiency disease (13).
Accordingly, the project aimed to assess the expression levels of SOCS1, IL-29, and LYST in patients with severe COVID-19 compared to healthy controls. The hypothesis was that the increased expression of anti-viral molecules by IL-29 and the involvement of LYST in intracellular vesicle movement might contribute to the induction of excessive inflammation in severe COVID-19 patients. Furthermore, the qualities of immune responses are influenced by two important factors, namely age and sex (14, 15), the mRNA levels of SOCS1, IL-29, and LYST were also compared among male and female patients as well as different age groups.
2. Objectives
The objective of this study was to evaluate the mRNA expression levels of SOCS1, IL-29, and LYST in patients who were infected with SARS-CoV-2 and exhibited severe symptoms.
3. Methods
3.1. Subjects
This cross-sectional study included a total of 70 healthy Iranian individuals without SARS-CoV-2 infection, who served as controls, and 70 SARS-CoV-2-infected patients with severe symptoms. Accordingly, mRNA levels of SOCS1, IL-29, and LYST were explored in the patients and compared to healthy controls. Patients contain 32 men and 48 women, and the controls were 35 men and 45 women, and the difference is not significant (P = 0.234). The groups were also not different in terms of age (P = 0.854). Accordingly, the patients were 50 ± 10 years old, and the controls were 49 ± 9 years old. The patients were positive for SARS-CoV-2 by real-time PCR, which were performed on a nasopharyngeal specimen and taken at the beginning of hospitalization and before treatments. The hospitalized patients at Afzalipour Hospital, Kerman, Iran, were included in this project. The healthy controls were free of any symptoms of the bacterial and viral respiratory diseases, and negative PCR for SARS-CoV-2 infection. The severity of the SARS-Cov-2-infection were evaluated and confirmed by an expert physician in infection according to the clinical symptoms and paraclinical data, Sonography and chest X-ray. The patients did not suffer from underlying disease, such as diabetes and heart diseases. To evaluate the mRNA levels of SOCS1, IL-29, and LYST, the blood samples were taken in anti-coagulant pre-treated tubes. The project protocol was approved by the Ethical Committee of Islamic Azad University, Kerman Branch (ethical code: IR.IAU.Kerman.REC.1400.028), and before blood collections, the participants filled out a written informed consent.
3.2. SARS-CoV-2 Detection
SARS-CoV-2 infection was assessed using real-time PCR. In accordance with this, three nasopharyngeal swabs were obtained from all participants and transferred to the molecular laboratory in viral transfer media. SARS-CoV-2 RNA was purified using a commercial kit (KPG, Iran). The N and RDRP genes of SARS-CoV-2 were detected using a commercial kit (KPG, Iran). As an internal control, RNase P was detected in the yellow channel of this kit.
3.3. Evaluation of Cytokine Signaling 1, Interleukin-29, and Lysosomal Trafficking Regulator mRNA Levels
Whole blood samples were collected using tubes containing an anti-coagulant, and total mRNA was purified from the collected samples using a commercial kit (KPG, Iran). Briefly, 200 microliters of the whole blood were added to 500 microliters lysis buffer and after 5 minutes of incubation at room temperature, precipitation buffer was added, and then transferred to a silica membrane column, and centrifuged at 8000 rpm for 1 minute. The column was washed, 50 microliters DNase-I were added, and it was incubated for 5 minutes at 37°C. The column was washed twice and then the DNase/RNase free water was used to purified total mRNA. After mRNA extraction, cDNA was synthesized using a commercial kit (KPG, Iran). The levels of SOCS1, IL-29, and LYST were assessed using the real-time PCR technique. To perform real-time PCR, the following material was added to 0.1 RotorGene microtubes: 3 microliters cDNA, 10 microliters SYBR Green master mix (Biosystem, England), 1 microliter primer (forward and reverse), and 6 microliters DNase/RNase/ free water at final 20 microliters. A two-channels RotorGene vehicle was used to amplify the targets and beta-actin was used as the house-keeping gene. The 2-ΔΔCt formula was utilized to calculate the expression ratio of SOCS1, IL-29, and LYST in the patients to the healthy controls (6).
3.4. Statistical Analysis
Student's t-test and Pearson correlation test, under SPSS software version 21, were used to compare the data between patients and healthy controls, male and female, and the correlations among the variables, respectively. The data are presented as mean ± standard error (SE). The protocol of the choosing of statistical tests were as our previous investigation (16).
4. Results
4.1. Relative Expression of Interleukin-29, Lysosomal Trafficking Regulator and Cytokine Signaling 1 in the Patients and Controls
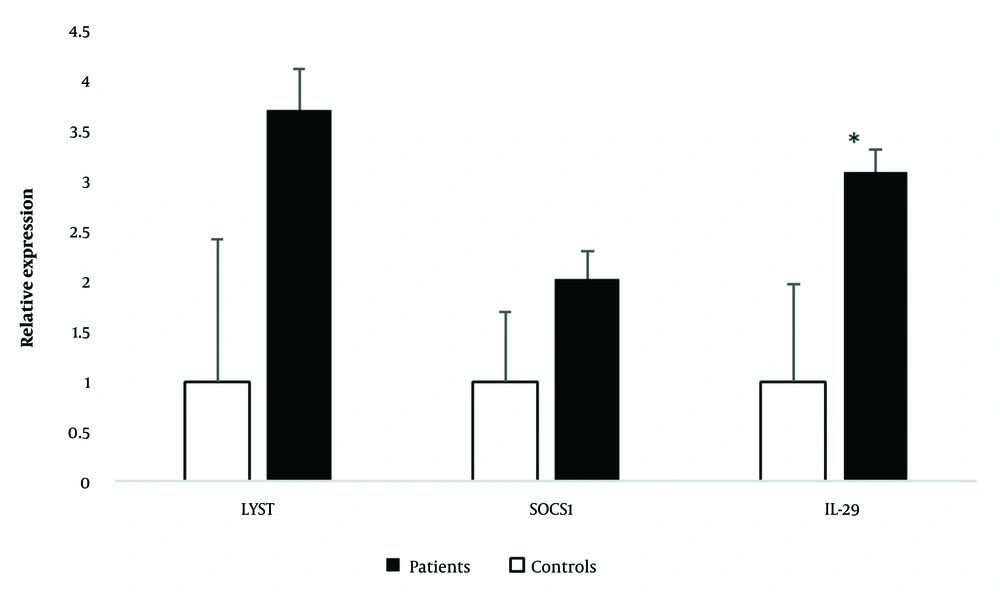
The student t-test revealed a significant increase in mRNA levels of IL-29 (P = 0.042) in severe SARS-CoV-2 infected patients compared to healthy controls. Accordingly, the relative expressions of IL-29 were 3.10 ± 0.97 in the patients and 1.00 ± 0.22 in the healthy controls (Figure 1).
The relative expression of interleukin-29 (IL-29), suppressor of cytokine signaling 1 (SOCS1), and lysosomal trafficking regulator (LYST) in the severe SARS-CoV-2 infected patients and healthy controls. IL-29 (*) significantly increased in the severe SARS-CoV-2 infected patients in comparison to healthy controls.
Although the mRNA levels of LYST (P = 0.073) and SOCS1 (P = 0.469) showed an increase in severe SARS-CoV-2 infected patients compared to healthy controls, the statistical analysis indicated that these differences were not statistically significant (Figure 1).
4.2. Sex Has No Effects on the mRNA levels of Interleukin-29, Lysosomal Trafficking Regulator, and Cytokine Signaling 1
The analysis of mRNA levels of IL-29, LYST, and SOCS1 in males and females, both in severe SARS-CoV-2 infected patients and controls, showed no significant differences. Table 1 presents the detailed mRNA levels of IL-29, LYST, and SOCS1 in males and females within each group.
| Variables | Patients | Controls |
|---|---|---|
| IL29 | ||
| Men | 3.55 ± 2.86 | 0.92 ± 0.22 |
| Women | 3.47 ± 1.15 | 1.05 ± 0.36 |
| P-value | 0.975 | 0.795 |
| SOCS1 | ||
| Men | 1.98 ± 1.47 | 1.48 ± 0.58 |
| Women | 2.40 ± 0.93 | 0.67 ± 0.23 |
| P-value | 0.820 | 0.179 |
| LYST | ||
| Men | 3.96 ± 3.84 | 0.38 ± 0.14 |
| Women | 4.35 ± 1.76 | 1.49 ± 0.69 |
| P-value | 0.918 | 0.188 |
Abbreviations: LYST, lysosomal trafficking regulator; IL29, interleukin-29; SOCS1, suppressor of cytokine signaling 1.
The student t-test revealed that mRNA levels of IL-29, LYST, and SOCS1 were not different between male and female participants in both severe SARS-CoV-2 infected patients and healthy controls.
4.3. Correlation Among mRNA Levels of Interleukin-29, Lysosomal Trafficking Regulator, and Cytokine Signaling 1, and Age
Table 2 displays the results of the Pearson correlation analysis assessing the relationships between mRNA levels of IL-29, LYST, and SOCS1, and age in both severe SARS-CoV-2-infected patients and healthy controls. The analysis revealed that there were no significant correlations between mRNA levels of these molecules and age in either the patient group or the healthy controls.
| Variables | Age Patients | Age Controls |
|---|---|---|
| IL29 | ||
| Pearson correlation | 0.085 | 0.072 |
| P-value | 0.736 | 0.908 |
| SOCS1 | ||
| Pearson correlation | 0.265 | 0.024 |
| P-value | 0.287 | 0.970 |
| LYST | ||
| Pearson correlation | 0.091 | 0.474 |
| P-value | 0.718 | 0.526 |
Abbreviations: LYST, lysosomal trafficking regulator; IL29, interleukin-29; SOCS1, suppressor of cytokine signaling 1.
The statistical analysis revealed that mRNA levels IL-29, LYST, and SOCS1 had no correlations with age in both patients and controls.
5. Discussion
Cellular immunity, also known as cell-mediated immunity, is a critical component of the immune response against viral infections (17). SARS-CoV-2 primarily targets and infects respiratory epithelial cells (18). Once inside the host cells, the virus replicates and spreads, leading to the release of viral particles (18). This triggers the immune system's response, including the activation of cellular immunity (16). Understanding the interactions between the human immune system and SARS-CoV-2 is crucial for developing effective treatments and interventions to modulate the immune response and prevent excessive inflammation (19, 20). Overall, cellular immunity plays a critical role in controlling and eliminating SARS-CoV-2 infection by directly targeting and eliminating virus-infected cells (17). Understanding the interplay between the virus and the cellular immune response is important for developing effective treatments and vaccines against COVID-19 (21). Interleukin-29, which is known as IFN-Lambda, plays several roles against viral infections (22). For example, it has been demonstrated that IL-29 can upregulate MHC class I, the main MHC for the presentation of cytoplasmic viral antigens to recognize by cytotoxic T lymphocytes (22). Upregulation of IL-29 during infection of respiratory tract epithelial cells by viruses has been demonstrated previously (23). Based on the findings of this study, it appears that after the infection of respiratory tract epithelial cells by viruses, there is an upregulation of IL-29. This upregulation of IL-29 can subsequently induce immune responses against the viruses, including SARS-CoV-2 (24). These immune responses may play a crucial role in combating the viral infection and mitigating its severity. According to our findings, it is evident that IL-29 may play a significant role in the promotion of excessive inflammation in severe cases of COVID-19. The three-fold increase in IL-29 expression among patients with severe COVID-19 further supports this hypothesis, highlighting IL-29 as a key contributor to the development of severe inflammation in COVID-19 pathogenesis. Fallah Vastani et al. reported a negative correlation between IL-29 serum levels and mortality in patients infected with SARS-CoV-2 (24). To our knowledge, the study conducted by Fallah Vastani et al. (24) is the only published study that has specifically investigated the expression of IL-29 in patients infected with SARS-CoV-2. However, it is important to note that their study focused on comparing the expression of IL-29 between surviving and deceased patients with SARS-CoV-2 infection. Our project aimed to compare SARS-CoV-2-infected patients with severe symptoms to healthy controls. Through our research, it became evident that further investigations are required to fully elucidate the key roles played by IL-29 during severe cases of COVID-19. Based on our hypothesis, we propose that IL-29 is responsible for inducing the expression of key molecules that act against SARS-CoV-2, thereby limiting its replication. Moreover, overexpression of IL-29 may contribute to dysregulated inflammation, which is a major driver of morbidity and mortality in COVID-19 (25). Therefore, we hypothesize that targeting IL-29 may potentially serve as a strategy to control inflammation in severe cases of COVID-19. While SOCS1 is known as a crucial regulator of cytokine signaling and immune responses (9), its role in the pathogenesis of severe COVID-19 remains a topic of debate. In our study, we observed that the mRNA levels of SOCS1 were not significantly altered in severe COVID-19 patients compared to healthy controls. However, a study conducted by Ahmed et al. demonstrated that degradation of SOCS1 could be linked to reduced replication of SARS-CoV-2 in an in vitro condition. Another review article proved the role played by SOCS1 in regulation of immune responses during COVID-19 (26). These contrasting findings suggest that the involvement of SOCS1 in severe COVID-19 requires further investigation and clarification (27). Indeed, the cell source of SOCS1 expression is an important factor to consider when studying the effects of SARS-CoV-2 infection (27). Kunnumakkara et al., observed that the expression of SOCS1 in human keratinocytes was decreased upon infection with SARS-CoV-2 in an in vitro condition. This finding suggests that the impact of SARS-CoV-2 on SOCS1 expression may vary depending on the specific cell type being investigated. Further exploration of SOCS1 expression in different cell types could provide valuable insights into its role in the context of SARS-CoV-2 infection (28). Another investigation showed that SOCS1 plays a crucial role in inhibiting immune responses against SARS-CoV-2 (26). This indicates that SOCS1 serves as an important molecule in regulating the immune system's reaction to the virus (26). By inhibiting immune responses, SOCS1 may affect the magnitude and duration of the immune reaction, potentially influencing the outcome and severity of COVID-19. More investigations are needed to fully understand the complex interactions between SOCS1 and the immune response during SARS-CoV-2 infection.
Additionally, the results demonstrated that relative expression of LYST were not changed in the patients when compared to healthy controls. Lysosomal trafficking regulator is a key molecule in the movement of immune cell granules and induction of appropriate immune responses (29). Our results showed that the molecule did not change in the patients and proposed that the molecule did not participate in the SARS-CoV-2-related inflammation. Therefore, it may be hypothesized that the molecule cannot considered for future molecular therapy against this virus. To the best of our knowledge, our project is the first study for the evaluation of the molecule in the patients; hence, it seems that more investigations are needed to confirm our hypothesis.
5.1. Conclusions
In light of these results, our study supports the hypothesis that IL-29 may play a role in severe inflammation during COVID-19. This suggests that IL-29 could potentially be explored as a molecular therapy for the treatment of severe and life-threatening cases of COVID-19. However, the roles played by SOCS1 and LYST in the pathogenesis of COVID-19 are suspected and additional investigations are needed to fully understand the mechanisms and potential therapeutic applications of these molecules in the context of severe COVID-19.