1. Background
Farmers use a variety of pesticides to kill plant pests to increase their crops (1). Early harvesting of agricultural products has led to the remaining toxins in the products, especially vegetables and fruits, entering the human body by consuming them (2). Diazinon is an insecticide frequently used in farming and widely used to control pests, which can be very toxic to animals and humans (3, 4) as of the organophosphorus compounds in agricultural chemicals (5). This poison is available in the market under the brand names aflatox, bazudyn, dassel, and gardentox. These compounds inhibit cholinesterase activity by phosphorylating the catalytic site of enzymes (6). Diazinon enters the body by direct inhalation exposure or skin contact, as most organophosphates can be absorbed through the skin (7). Organophosphate compounds have toxic effects on the reproductive system in male rats. According to histology, they cause severe focal necrosis or degeneration of germ cells in spermatogenic tubules associated with atrophy (8, 9). Ahmadi et al. reported that diazinon toxin induces free radical production and causes oxidative stress in a dose-dependent manner (10). The change in enzyme activity and decrease in glutathione concentration is due to the reduction of the antioxidant defense system against free radicals and tissue oxidative damage (5). Antioxidants such as quercetin can be beneficial for neutralizing free radicals produced by diazinon and reducing oxidative stress. Quercetin is a carotenoid extracted from the extract of the plant Crocus sativus L of the Iridaceae family. Research has shown that quercetin has antioxidant activity in laboratory conditions, which reduces the level of malondialdehyde (MDA) molecule and the increase in the level of ferric-reducing ability of plasma (FRAP) found following kidney ischemia-reperfusion stress (11).
2. Objectives
The most commonly found toxin in human foods is diazinon, which is an organophosphate toxin. The accumulation of diazinon in the human body can be observed through food, breathing, and even skin absorption. Diazinon poison negatively affects various organs of the body. The use of antioxidants can be effective in reducing the side effects of diazinon (12). Quercetin, as an antioxidant against toxins, has attracted the attention of many researchers. Therefore, the present research aimed to evaluate the protective role of quercetin on the reproductive tissue of rats treated with diazinon.
3. Methods
3.1. Preparation of Chemicals
Diazinon (purity of 100%) from SupelcoUSA and quercetin (purity ≥ 98%; CAS No. 117 - 39 - 5) from the Sigma-Aldrich Company Ltd were purchased. Fresh stock solutions of diazinon were prepared in corn oil. In contrast, the quercetin solution was prepared based on established procedures (dissolved in distilled water, homogenized at 36°C for 30 minutes) as outlined in the referenced literature (13).
3.2. Experimental Animals
In this research, 40 adult male rats were purchased and used from Uremia University Animal House. The weight of each rat was between 110 – 160 g. All animals were healthy in appearance, and they were kept without any intervention for a week before the start of the experiment to acclimatize the animals to the laboratory environment. The housing conditions (temperature between 20 - 230 c, 12 hours of light, and 12 hours of darkness) were strictly controlled. Food and water were freely available to the animals. The animals were divided into five groups of eight rats: Sham-control, diazinon, experimental 1, experimental 2, and experimental 3. The animals received respective doses of corn oil in the amount of 0.2 mL, diazinon 300 mg/kg, diazinon 300 mg/kg quercetin 25 mg/kg, diazinon 300 mg/kg and quercetin 50 mg/kg, diazinon 300 mg/kg and quercetin 100 mg/kg orally daily by gavage for 60 days. This research was conducted based on the instructions of the Ethics Committee in Uremia University Faculty of Veterinary Medicine (Ref: IR-UU-AEC.3/3-8.4.2024).
3.3. Testicle Weight Assays
At the end of the period, all animals were anesthetized with xylazine (5 mg/kg) and ketamine 5% (40 mg/kg). Then, animals were killed by Trans locating the cervical vertebrae immediately after weighing the whole body. Blood was collected from the rat's hearts to check the amount of sex hormones. Then, the rats were dissected, and the testicles were removed from each rat and weighed.
3.4. Histochemical Assays
The testes were stable in 10% formalin and then inserted in paraffin for histological evaluation. Sections were prepared from the testicular tissue and stained with Iron-Weigert to identify the nucleus of germ cells in the testicle for histopathological evaluation. All samples were studied at multiple magnifications. Dimensions were expressed in micrometers. Partial testicles were used fresh for histochemical examination. Therefore, frozen slices of 15 µm were prepared from the samples. Periodic acid Schiff (PAS) staining was used to check the carbohydrate reserves and Sudan block-B to lipid in testicular tissue.
3.5. Tubular Differentiation Index, Repopulation Index, and Sperm Index Assays
The percentage of testicular tubes determines the tubule differentiation index (TDI) with more than three cell layers and the percentage of spermatogenic tubules with less than three.
The ratio of type B spermatogonia to type A spermatogonia was counted in the spermatogenic tubes to determine the repopulation index (RI).
The number of testicular spermatogenic tubules without sperm inside their lumen was counted and expressed as a percentage to determine the sperm index (SI).
3.6. Ferric-Reducing Ability of Plasma and Malondialdehyde Assays
The FRAP of the control sham and experimental groups was measured to measure the protective effect of quercetin on oxidative stress. This method evaluated the acid pH created by the acetate buffer and the Fe III -TPTZ complex's reduction effect on the ferrous form. The complex is colored blue in the presence of the acetate buffer. About 100 µL is taken, added to 3 mL of FRAP reagent from the testis tissue solution, and put in the incubator for 8 minutes. The absorbance of the blue complex was measured at 593 nm (14).
Then, 0.3 g of testicular tissue was prepared in potassium chloride and centrifuged at 3000 × g for 10 minutes to evaluate MDA. About 3 mL of phosphoric acid was mixed with 0.5 mL of supernatant, then 2 mL of 6.7 g/L TBA was added. The samples were placed at 100°C for 45 minutes and then cooled. Finally, 3 mL n-butanes were added, and the samples were centrifuged for 10 minutes. The supernatant liquid was measured by spectrophotometric method at 532 nm wavelength and MDA amount (15).
3.7. Hormonal Assays
After the experimental period ended, blood was collected from the animals' hearts to separate the serum. The samples were centrifuged, and the collected serums were analyzed. The electrochemical luminescence method was used to measure the testosterone hormone.
3.8. Statistical Analyses
The data obtained from this research were analyzed using one way ANOVA. Turkey's test was used to determine the difference between groups, and P < 0.05 was considered as the level of significant difference between groups. The obtained values were reported as mean standard deviation. SPSS No. 19 software was used for analysis.
4. Results
4.1. Histology
Statistical analysis of the data showed that the weight loss of diazinon and experiment 1 group was significant compared to the control-sham group (P < 0.50), but the reduction of body weight in experimental group 1 is not significant compared to diazinon (P > 0.05). In addition, experimental groups 2 and 3 significantly increased compared to diazinon (P < 0.50) (Table 1).
| Parameters | B.W.G (g) | (BWG/TW) | TDI (Negative) (%) | RI (%) | SI (%) |
|---|---|---|---|---|---|
| Control-sham | 198 ± 0.12 A | 47.5 ± 2.1 A | 5.96 ± 0.2 A | 1.52 ± .84 A | 3.87 ± .25 A |
| Diazinon | 151 ± 2.13 B | 68.1 ± 2.84 B | 21.3 ± 0.75 B | 0.41 ± 1.2 B | 20.41 ± 2.3 B |
| Experimental 1 | 156 ± 1.15 B | 70.1 ± 3.12 B | 19.01 ± 0.32 B | 0.51 ± 2.1 B | 18.17 ± 1.6 B |
| Experimental 2 | 168 ± 1.32 A | 59.3 ± 0.63 A | 7.22 ± 0.2 A | 0.85 ± 3.50 B | 5.30 ± 0.32 A |
| Experimental 3 | 172 ± 2.22 A | 56.7 ± 0.91 A | 6.22 ± 0.2 A | 1.42 ± 1.32 A | 4.16 ± 0.82 A |
| F-value | 321.05 | 74.659 | 263.531 | 2428.253 | 625706.3 |
| df | 4 | 4 | 4 | 4 | 4 |
| Sig | 0.001 | 0.002 | 0.000 | 0.001 | 0.000 |
Abbreviations: TDI, tubule differentiation index; SI, sperm index; RI, repopulation index.
a Values are expressed as mean ± SD unless otherwise indicated.
b In each column, there is no significant difference between groups with the same superscript letters (P > 0.05), but there is a significant difference between groups with different superscript letters (P < 0.50).
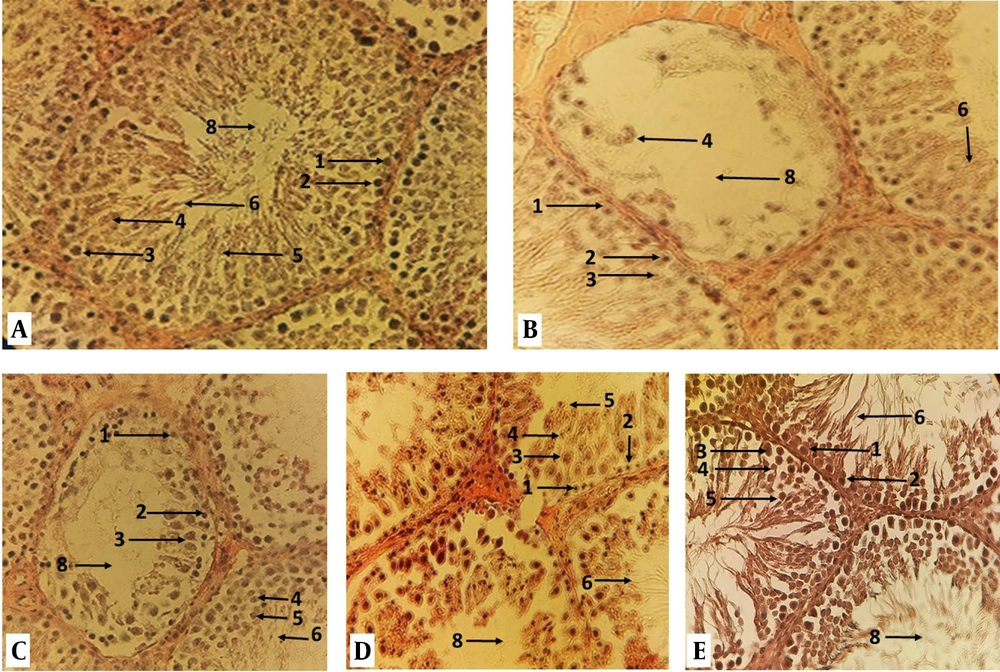
The result of the TDI and SI showed that the percentage of spermatogenic tubule differentiation index and spermiogenesis index of the diazinon group significantly increased compared to the control-sham (P < 0.50), but it decreased significantly in experimental group 2 and 3 compared to diazinon group (Table 1, Figure 1).
The testicular tissue: A, control-sham; B, diazinon; C, experimental 1; D, experimental 2; and E, experimental 3 groups rat, where tubule differentiation index (TDI), repopulation index (RI), and sperm index (SI) can be seen in Iron-Weigert staining. (1) Spermatogoni B; (2) spermatogonia A; (3) primary spermatocyte; (4) secondary spermatocyte; (5) spermatid; (6) sperm; (7) leydig cells; (8) lumen of the spermatogenic tube (magnification 400 ×).
The results related to the replacement index of spermatogonia (RI) showed that the ratio of type B spermatogonia to type A (RI) diazinon group compared to the control was significantly decreased (P < 0.50), but experimental group 3 significantly increased compared to the diazinon group (P < 0.50) (Table 1, Figure 1).
4.2. Histopathological
Histological studies showed that the animals in the diazinon group had significantly degenerated testis, with significant edema and atrophy in the spermatogenic tubules and tissue showing interstitial ligament, while quercetin significantly reduced atrophy and edema (Figure 1).
4.3. Cellular Carbohydrate Reserves
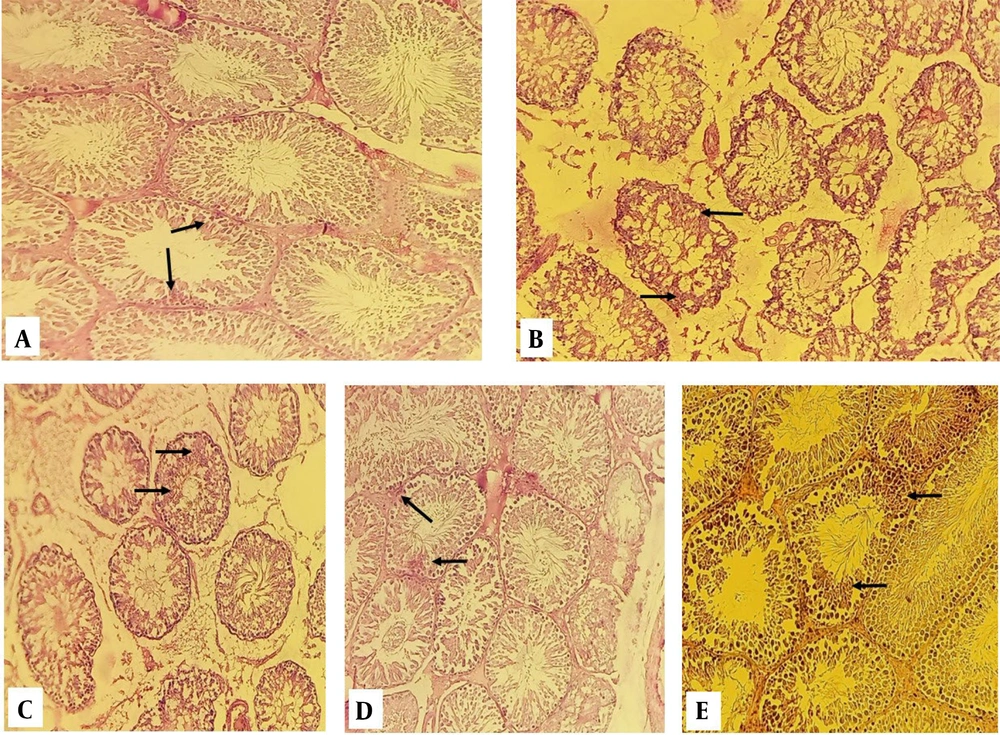
Assessment of the results by light microscope revealed that in the control-sham, the PAS reaction was strongly positive in the spermatogenic tubules and interstitial cells' wall cells (Figure 2A). A weak positive PAS reaction was observed in interstitial and spermatogenic cells in the diazinon (Figure 2B). In experimental 1, the PAS reaction in the cells of the seminiferous tubules was moderate (Figure 2C). The PAS reaction in experimental groups 2 and 3 was relatively strong (Figure 2D, E).
Section of the testis in periodic acid Schiff (PAS) staining; A, control-sham group; the cells inside the spermatogenic tubules show a solid reaction to PAS staining, but in diazinon group; B, remained stainless; C, in the experimental 1 group, the cells inside of the spermatogenic tubules show a weak reaction to PAS staining; D and E, experimental groups 2 and 3 show a relatively strong reaction to PAS staining.
4.4. Cellular Lipid Reserves
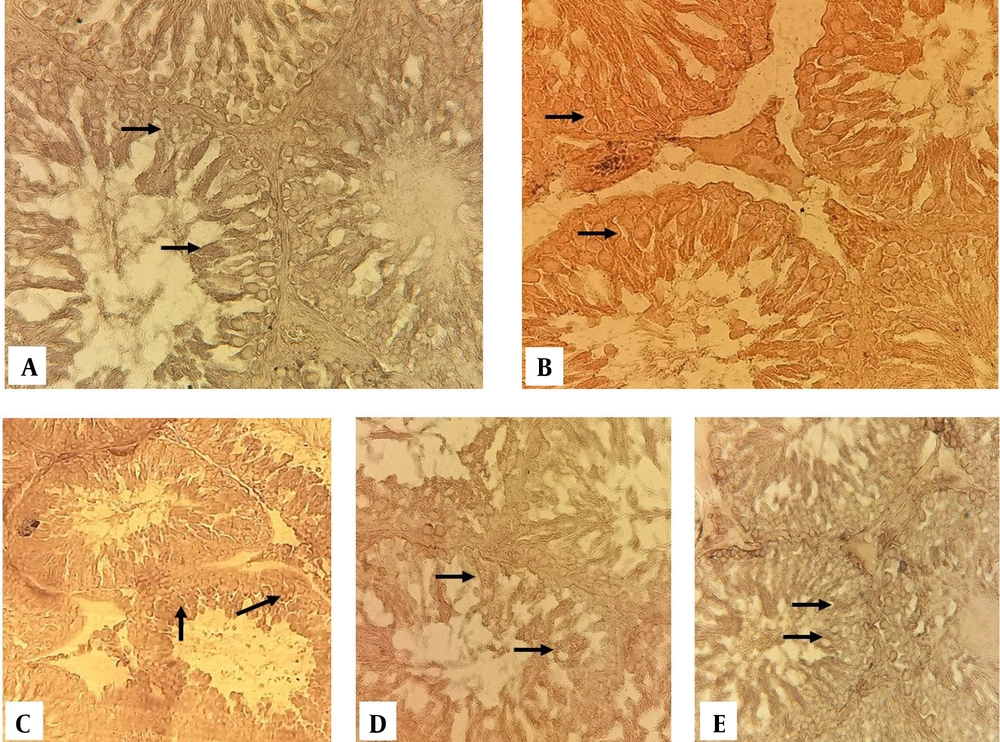
Histochemical investigation related to the concentration of cytoplasmic lipids revealed that the amount of lipid reserves in the first rows of the germinal layer of the testis of control-sham rats is very weak (Figure 3A). In addition, the diazinon and experimental one-group of spermatogenic tubule wall cells showed a strong reaction to Sudan block-B staining (Figure 3B and C). In experimental groups 2 and 3, cells of the spermatogenesis series showed a relatively normal reaction to Sudan block-B staining (Figure 3D and E).
Cross section from testes Sudan black-B staining (400 ×); A, control-sham group; the cells inside the spermatogenic tubules show a weak reaction to Sudan block-B staining, but the reaction of the cells in the diazinon group B and C is strong; D and E, in experimental groups 2 and 3, the spermatogenesis cell series are presented as approximately normal.
4.5. Oxidative Stress and Testosterone Level
Assessment results of oxidative stress revealed that the amount of MDA molecule in the diazinon group and experimental 1 compared to the control-sham significantly increased (P < 0.50) but in experimental groups 2 and 3 compared to control-sham not significantly increase (P > 0.50). The result of the study revealed that the amount of ferric reducing ability of plasma (FRAP) in diazinon, experimental 1 and 2 groups in compared to control-sham significantly decreased (P < 0.50), but the amount of FRAP of the experimental groups 3 in compared to control-sham not decreased considerably (P < 0.50) (Table 2).
| Parameters | FRAP (nmol/mL) | MDA (nmol/mg) | Testosterone (ng/mL) |
|---|---|---|---|
| Control-sham | 2.3 ± 1.6 A | 0.46 ± 0.08 A | 5.3 ± 0.5 A |
| Diazinon | 0.88 ± 0.36 B | 2.3 ± 0.06 B | 3.71 ± 0.6 B |
| Experimental 1 | 1.07 ± 0.87 B | 2.01 ± 0.04 B | 4.1 ± 3.1 B |
| Experimental 2 | 1.28 ± 1.64 B | 1.06 ± 0.6 A | 4.9 ± 045 A |
| Experimental 3 | 2.01 ± 0.11 A | 0.87 ± 0.12 A | 5.1 ± 0.62 A |
| F-value | 91.882 | 307.079 | 54.929 |
| df | 4 | 4 | 4 |
| Sig | 0.001 | 0.000 | 0.010 |
Abbreviations: FRAP, ferric-reducing ability of plasma; MDA, malondialdehyde.
a Values are expressed as mean ± SD unless otherwise indicated.
b In each column, there is no significant difference between groups with the same superscript letters (P > 0.05), but there is a significant difference between groups with different superscript letters (P < 0.50).
The analysis of testosterone hormone data revealed that the amount of testosterone hormone in the diazinon group and experimental one significantly reduced compared to the sham control (P < 0.50). While in experimental groups 2 and 3, compared to the control sham, there was no significant decrease (P < 0.50) (Table 2).
5. Discussion
Diazinon is an organophosphorus compound mainly used in agricultural and non-agricultural environments. These compounds phosphorylate the catalytic site of the cholinesterase enzyme, preventing its activity and causing damage to the male reproductive system (16).
The study's results showed that diazinon causes severe degeneration of spermatogenic tubules. In contrast, animals that received quercetin showed tissue-reconstructed spermatogenic tubules. The possible mechanism is that diazinon has caused severe inflammation in tubules, increased oxidative stress, and induced apoptosis in spermatogenic series cells. Thus, significant cellular depletion occurred in the tubules after acute apoptosis (16).
The research results indicated a significant decrease in the RI index among rats treated with diazinon. Conversely, animals administered quercetin exhibited normal parameters. It is hypothesized that spermatogonial cells are highly susceptible to diazinon, which may inhibit the repopulation ratio within the tubules by inducing tissue inflammation, leading to a marked reduction in the active cell population. Consequently, this pathological mechanism halts sperm production in most tubules. However, in animals treated with quercetin, the progression of the inflammatory response was mitigated, and the tissue of the spermatogenic tubules remained normal.
According to a former study, glucose is the chief energy source for the spermatogenesis cell to produce essential proteins (17). Any change caused by diazinon can interrupt glucose metabolism because glucose transporter proteins are the main elements of glucose transport into spermatogenesis cells (18).
The histochemical analysis revealed that spermatogenic cells in the diazinon group exhibited weak cytoplasmic carbohydrate accumulation. In contrast, animals treated with quercetin showed a significant increase in carbohydrates. The spermatogenesis cells may have shifted from utilizing glucose to lipids due to the loss of energy sources in the diazinon group. Sudan black- B staining was employed to investigate these potential changes. The results demonstrated that animals receiving diazinon alone exhibited a solid reaction to Sudan black staining, whereas the animals receiving both diazinon and quercetin showed a weaker reaction. Consequently, it can be inferred that by reducing free radicals, quercetin mitigates oxidative stress and inflammation, allowing cells to derive energy from carbohydrate sources.
The current study’s findings indicate that testosterone levels in the diazinon group were lower compared to the control. Conversely, animals treated with both quercetin and diazinon exhibited elevated serum testosterone levels. Research has demonstrated that Leydig cells’ testosterone production is contingent upon gonadotropin hormone (19). Additionally, most organophosphorus compounds suppress Leydig cells’ activity, culminating in diminished testosterone synthesis (20). Consequently, diazinon exposure may precipitate Leydig cells’ degeneration, a decline in blood testosterone levels, and, subsequently, compromised spermatogenesis.
Research findings showed that in the diazinon group, compared to the control, the concentration of MDA molecule increased significantly, while the concentration of FRAP molecule significantly decreased. In contrast, experimental groups 2 and 3 showed a significant decrease in MDA levels and an increase in FRAP levels compared to the diazinon group alone. The possible causes of MDA increase are damage to the sperm cell membrane, reactive oxygen species (ROS) production, and increased oxidative stress. Due to its antioxidant properties and role in neutralizing free radicals, quercetin has reduced these molecules in cells and reduced oxidative stress. These findings are consistent with the findings reported by Khayatnouri et al. in 2011 (21).
Consequently, it can be concluded that quercetin protects rat testis tissue against the effects of diazinon through its antioxidant activity.