1. Background
Wound healing is a complex biological process involving various cellular and molecular mechanisms to restore tissue integrity and function (1). Understanding the intricate interplay of these mechanisms is crucial for developing effective therapeutic strategies to enhance wound healing and mitigate associated complications (1, 2). Recently, atmospheric cold plasma (ACP) has emerged as an effective technique for promoting wound healing due to its ability to produce reactive oxygen and nitrogen species and electromagnetic fields, which can modulate tissue repair cellular responses (3). Atmospheric cold plasma is an ionized gas containing various reactive species, including ions, electrons, radicals, and UV photons (4). Atmospheric cold plasma can be generated at room temperature and atmospheric pressure, making it suitable for biomedical applications. The unique composition of ACP allows it to exert antimicrobial, anti-inflammatory, and pro-regenerative effects, making it an attractive modality for wound healing applications (5). Atmospheric cold plasma has been shown to stimulate essential processes for efficient wound repair, including angiogenesis, enhance epithelialization, and promote collagen synthesis (6). Additionally, ACP has demonstrated potent antimicrobial activity against various pathogens, including bacteria, fungi, and viruses, reducing the risk of wound infection and accelerating healing (5).
Among the various gases used in ACP technology, argon, and helium have received attention considerably due to their unique properties and potential therapeutic effects (7). As an inert gas abundant in the earth's atmosphere, argon has proven to be remarkably effective at penetrating tissues (8). A deeper wound can be healed with argon plasma because of its inert nature, enabling deeper wounds to be healed without significant damage (9). Moreover, argon has exhibited potent anti-inflammatory properties, capable of mitigating excessive inflammation, a common impediment to efficient wound repair (8). Despite being lighter than argon, helium's advantages can also be found in wound healing applications. Helium plasma jets, characterized by their high energy and precision, have demonstrated superior tissue penetration compared to other gases (10). This enhanced tissue penetration allows helium plasma to exert its therapeutic benefits more effectively, targeting specific cellular and molecular pathways involved in wound repair (11). Although ACP is widely used for wound management, comparative studies still do not explain the differences in wound healing between argon and helium ACP (12, 13).
2. Objectives
Therefore, this study aimed to investigate the comparative impact of argon and helium atmospheric cold plasma jets on wound healing in a rat model.
3. Methods
3.1. Study Animals
A total of 18 male Wistar rats, weighing approximately 80 – 100 g each, were used in this study (9). All rats were housed under standardized conditions with ad libitum access to food and water, maintained on a 12-hour light-dark cycle at approximately 25°C in individual cages complying with sanitary standards. Rats were randomly assigned to three groups: Control (n = 6), helium ACP-treated (n = 6), and argon ACP-treated (n = 6).
3.2. Wound Creation Method
The study animals were first anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneal to create the wounds. Then, the area was thoroughly shaved and disinfected with povidone-iodine, and an 8mm full-thickness wound was made between the two shoulders of the samples using a biopsy punch.
3.3. Treatment Method in Groups
In the first group, cold atmospheric helium plasma, operating at a frequency of 50 kHz, a voltage of 8 kV, and a current of 30 mA, and supplied by Hoopad Sanat Plasma Company, was administered for 1 minute on days 0, 3, 7, 10, 14, 17, and 21 to the wounds of all samples in this group, with the nozzle positioned 1 cm away from the wound. In the second stage, wound dressing was performed with three layers (first layer sterile gauze, second layer Vibraplex, third layer band) after soaking the wounds with silver sulfadiazine ointment. Then, the dressing was covered with a layer of Locuplast adhesive to make it waterproof. The dressing was changed daily, and after each dressing removal, the wound was moistened with sterile gas, cleaned with washing serum, washed with sterile saline, and dried with sterile gas. Then, plasma radiation was performed on the specified days, and the dressing was performed again. In the second treatment group, in the first stage, cold atmospheric argon plasma with a frequency of 50 kHz, a voltage of 6 kV, and a current of 30 mA, with a nozzle distance of 1 cm from the wound, was applied for 1 minute on days 0, 3, 7, 10, 14, 17, and 21 to the wounds of all samples in this group. The second stage involved dressing the wounds after soaking them with silver sulfadiazine ointment and then covering the dressings with Locuplast adhesive as a waterproofing agent. Dressing change was performed daily, and the wound care protocol was followed as in the first treatment group. The wounds in the control group were only treated with silver sulfadiazine ointment without plasma irradiation. Dressing was performed similarly to the treatment groups, and wound care was done daily.
3.4. Wound Healing Analysis
After each plasma therapy session, one sample was chosen randomly from each group on days 1, 3, 5, 7, 14, and 21. The wound area was measured after removing the dressing and performing the necessary cleansing to assess the wound size. In addition, IL-6 levels were measured using ELISA kits from Karamania Pars Gene Company, and CBC tests were performed after CO2 anesthesia and blood from the heart was collected. Then, a full-thickness skin sample was taken from the skin surrounding the wound for pathological examination of the wound (9).
3.5. Macroscopic Examination
The wounds were examined through a macroscopic analysis using a ruler to measure their size. Samples were randomly selected from each group, and images of the wounds were captured after removing the dressing and cleaning for wound inspection. Digital images were analyzed using Digimizer software to measure the area of the wounds.
3.6. Microscopic Examination
Following anesthesia with CO2, skin samples measuring 15 × 15 mm in total thickness were collected from the skin surrounding the wounds. These samples were then fixed in 10% formalin, and relevant information about each sample was recorded on the container. Subsequently, the skin samples were sent to the laboratory for further processing. The following assessments were conducted after preparing histological sections and staining: Epithelial gap (µ), granulation tissue (µ2), angiogenesis (n/mm2), fibroblast cells (n/mm2), thickness of necrotic layer (µ), re-epithelialization (µh), hyperemia, and inflammation.
3.7. Statistical Analysis
All data were stored in Excel software and analyzed using SAS-9.4 software. Descriptive statistics were extracted, and initial statistical distributions were examined. The data normality distribution and variances' independence were assessed using the Kolmogorov-Smirnov test at a 5% significance level. One-way analysis of variance (ANOVA) was conducted to compare the measured indices between the three experimental groups, and Duncan's multiple range test was used to identify significant differences between means. A chi-square non-parametric test was also performed to compare qualitative indices among the three experimental groups.
4. Results
Table 1 indicates significant differences between the experimental groups for the hemoglobin (HGB), hematocrit (HCT), and mean corpuscular volume (MCV) parameters (P < 0.05). These results showed higher HGB and HCT in the experimental groups compared to the control group and lower MCV in the experimental groups compared to the control group. Regarding the other measured parameters, there was no significant difference between the experimental groups regarding overall means (P > 0.05). The level of PLT in the group treated with helium plasma was higher compared to other groups, but this difference was not significant.
| Variables | Control | Helium | Argon | SEM | P-Value |
|---|---|---|---|---|---|
| WBC | 9.83 | 9.28 | 12.38 | 1.13 | 0.54 |
| RBC | 5.75 | 7.42 | 6.97 | 0.34 | 0.12 |
| HGB | 13.71 | 15.78 | 15.60 | 0.35 | 0.02 |
| HCT | 37.06 | 45.72 | 44.85 | 1.82 | 0.03 |
| MCV | 69.03 | 62.23 | 64.50 | 2.08 | 0.02 |
| PLT | 509.00 | 549.67 | 491.17 | 23.86 | 0.61 |
| MCH | 21.90 | 21.36 | 22.43 | 0.32 | 0.36 |
| MCHC | 34.64 | 34.40 | 34.77 | 0.18 | 0.72 |
Table 2 demonstrates that the levels of epithelial gap in the helium plasma-treated group, granulation tissue in the control group, angiogenesis in the argon plasma-treated group, thickness of necrotic layer in the control group, and re-epithelialization in the argon plasma-treated group were higher than in the other groups. The level of fibroblast cells was approximately equal in all three groups. However, none of these differences among the investigated groups were significant (P > 0.05).
| Variables | Control | Helium | Argon | SEM | P-Value |
|---|---|---|---|---|---|
| Epithelial gap (µ) | 3077 | 4640 | 4393 | 830.71 | 0.73 |
| Granulation tissue (µ2) | 4.02 × 106 | 3.55 × 106 | 3.61 × 106 | 0.65 × 106 | 0.95 |
| Angiogenesis (n/mm2) | 149.83 | 163.33 | 212.83 | 17.85 | 0.32 |
| Fibroblast cells (n/mm2) | 3326.7 | 3364.3 | 3164.2 | 375.19 | 0.97 |
| Thickness of necrotic layer (µ) | 246.7 | 220.0 | 220.8 | 49.05 | 0.97 |
| Re-epithelialization (µh) | 30.58 | 17.50 | 57.50 | 11.75 | 0.38 |
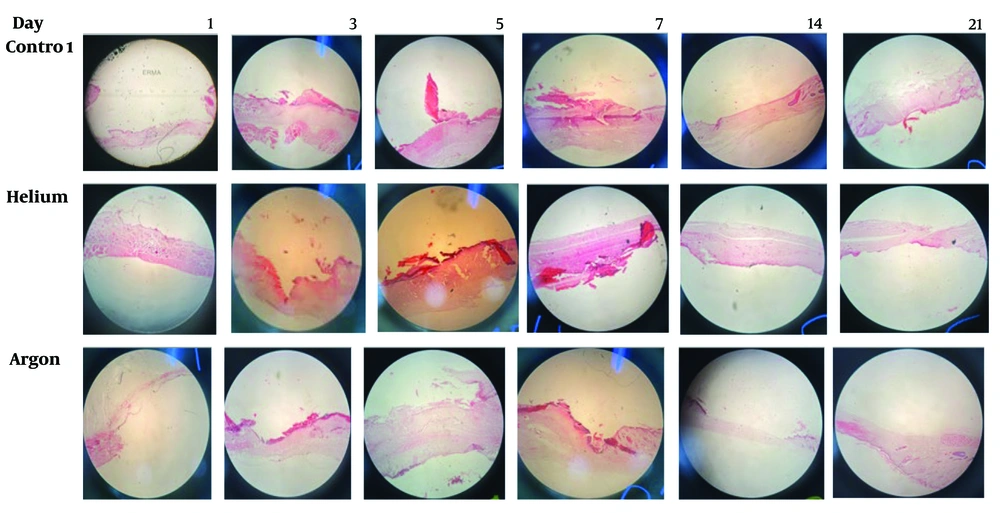
Table 3 shows significant differences in the samples with statuses -, +, ++, or +++ among the three experimental groups for the two parameters: Hyperemia and Inflammation (P < 0.01). The results indicated that no inflammation was observed in two samples of rats treated with helium plasma and in one sample of rats treated with argon plasma, but all samples exhibited some degree of inflammation in the control group. The level of hyperemia in the treatment groups with helium and argon plasma was generally higher than in the control group, with the argon plasma treatment group demonstrating higher degrees of hyperemia than the helium plasma treatment group. The level of inflammation in the treatment groups with helium and argon plasma was generally lower than the control group, with the inflammation degrees in the helium plasma treatment group being lower than those in the argon plasma treatment group (Figure 1).
| Variables and Grouping Observations | Control | Helium | Argon | P-Value |
|---|---|---|---|---|
| Hyperemia | 0.001 | |||
| - | 2 (33.3) | 1 (16.7) | 0 (0) | |
| + | 2 (33.3) | 2 (33.3) | 2 (33.3) | |
| ++ | 1 (16.7) | 3 (50) | 3 (50) | |
| +++ | 1 (16.7) | 0 (0) | 1 (16.7) | |
| Inflammation | 0.001 | |||
| - | 0 (0) | 2 (33.3) | 1 (16.7) | |
| + | 2 (33.3) | 1 (16.7) | 1 (16.7) | |
| ++ | 2 (33.3) | 0 (0) | 2 (33.3) | |
| +++ | 2 (33.3) | 3 (50) | 2 (33.3) |
a Values are expressed as No. (%).
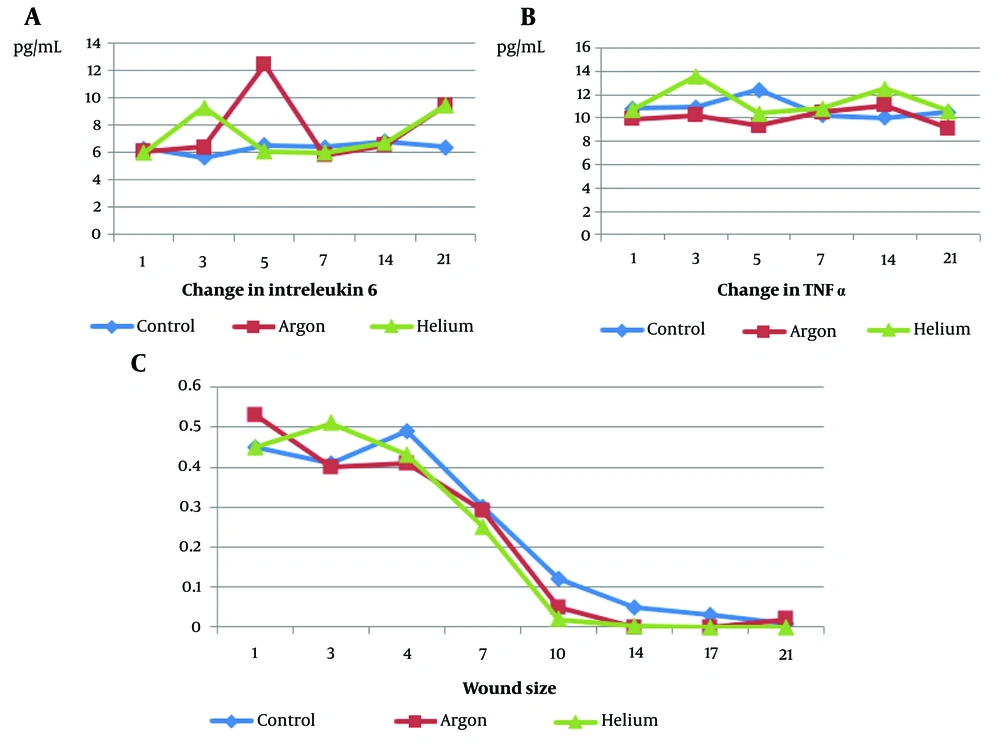
The results indicated that the level of tumor necrosis factor alpha (TNF-α) was highest in the helium experimental group and lowest in the argon group, with a significant difference between the two groups (P < 0.05). However, the control group did not show a significant difference in TNF-α levels compared to these two groups (P = 10.80). The changes in wound size between the experimental groups and the control group were insignificant during this period (P > 0.05), but the average wound size in the plasma helium-treated group was lower than the other two groups. The level of IL-6 in the plasma argon-treated group was higher compared to the other groups and lower in the control group compared to the other groups (Figure 2).
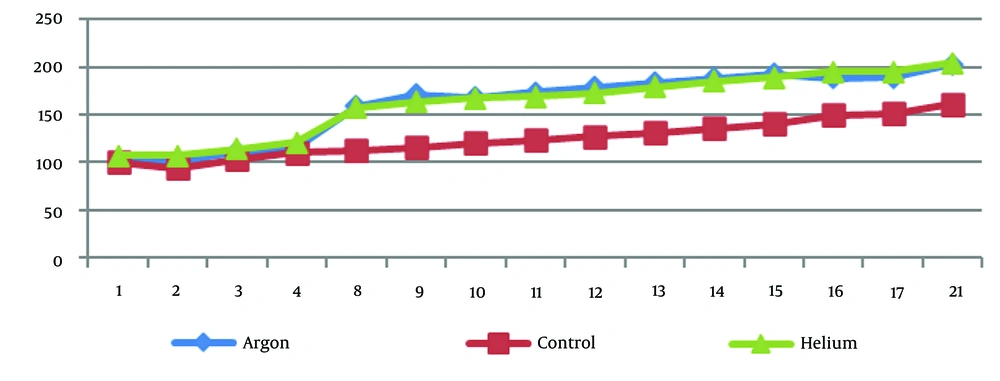
Figure 3 demonstrates that the weight of rats under treatment with argon and helium plasma has consistently been higher from day four onward than that of the control group.
5. Discussion
The present study aimed to compare the effects of cold atmospheric plasma jets with argon and helium on histopathological changes and biochemical markers in wound healing in rats. The results of this study showed that the TNF-α level in the group treated with argon was the lowest, and IL-6 had the highest level, while in the experimental group treated with helium, TNF-α had the highest level. Consistent with these results, Lee et al. demonstrated in a study focusing on the expression of wound healing genes in a shoulder burn induction model in rats that treatment with plasma jets significantly affects the genes crucial for the wound healing process, resulting in significant improvement. The results also indicated a decrease in pro-inflammatory cytokines such as TNF-α and IL-1β and an increase in anti-inflammatory cytokines such as Tgϯ-β, IL-10, and IL-6, which align with the findings of the present study (14).
Like Lee et al., (14) argon-treated rats had the lowest TNF levels and the highest levels of IL-6, whereas helium-treated rats had the highest TNF levels. The present study revealed that plasma irradiation led to improved wound-healing processes. Dang et al. analyzed the infected burn wounds in Syrian rats and the effect of cold argon plasma on wound size, inflammatory cytokines, anti-inflammatory macrophages, and fibroblasts. The results showed that non-thermal plasma treatment enhanced burn wound healing through several wound healing factors, including anti-inflammatory macrophages and improved fibroblast migration, consistent with the present study. That study recommended that using voltages below 10 kilovolts as higher amounts could potentially damage macrophages and weaken their function. However, significantly better results were achieved overall than the control group (15).
In the present study, there was no significant difference in wound size changes between the experimental groups and the control group during this timeframe, but the average wound area in the helium plasma-treated group was lower compared to other groups. Dorehlo et al., in a study on the burned skin of adult Syrian rats, found that the experimental groups had a significant increase compared to the control group in burn injuries. In addition, the number and diameter of skin vessels significantly increased compared to the control group. The wound area also significantly decreased on the seventh day compared to the control group. The results indicated that cold helium plasma radiation was effective in all wound healing assessment indices, and its application during burn injuries could accelerate wound healing (16).
Moreover, Dehghanpishe et al., in a comparative study on the histopathological effects of cold plasma and ultraviolet radiation on fully infected wounds in male desert rats, demonstrated that helium cold plasma and ultraviolet radiation had a positive effect on the presence of mononuclear inflammatory cells, collagen formation, and epithelial regeneration. According to this study, cold plasma and ultraviolet radiation could be employed in wound healing, with cold plasma exhibiting a more significant impact on improving wound healing, which is in line with the results of the present study (17).
The present study revealed that re-epithelialization was higher in the plasma-treated groups than in the control group, and the thickness of the necrotic layer was lower in these groups than in the control group. Overall, the results obtained were similar to those of Dorehlo et al. (16), indicating the significant impact of plasma radiation on the wound healing process. The current study demonstrated that angiogenesis and re-epithelialization were higher in the group treated with argon plasma than in other groups, and the thickness of the necrotic layer was lower in this group. Additionally, no signs of inflammation were observed in a sample of rats treated with argon plasma, while all samples in the control group exhibited some degree of inflammation. Furthermore, higher levels of hyperemia were observed in the group treated with argon plasma.
In this regard, Ale Ebrahim et al. evaluated the tissue pathological effect of cold argon plasma on atmospheric pressure on accelerating blood clotting speed and wound healing in full-thickness skin wounds in rats. The results demonstrated that plasma radiation increased blood clotting speed in cutaneous wounds in vivo. Based on the histopathological findings, rats in the treatment group with plasma showed a significant trend in full-thickness skin wound healing (18). Therefore, cold argon plasma can enhance blood clotting speed and improve the healing process in cutaneous skin wounds.
5.1. Conclusions
Based on the results, both argon and helium atmospheric cold plasma treatments demonstrated significant potential in modulating wound healing processes, as evidenced by their effects on cytokine levels and tissue pathology. Argon plasma showed promising results in reducing TNF-α levels and increasing IL-6 levels, indicating its potential to promote wound healing. Meanwhile, helium plasma exhibited efficacy in reducing wound size. Therefore, both types of cold plasma could be valuable therapeutic options for enhancing wound healing, with argon plasma potentially offering benefits in blood coagulation speed and overall tissue repair. Further research and clinical trials are suggested to explore the full therapeutic potential and optimal application of argon and helium cold plasma in wound management.