1. Background
Lung cancer (LC) is a non-communicable disease and remains the most common cancer worldwide (1). According to estimates from Globocan 2018, it is the most common cancer in both genders and leading cause of cancer-related death with 2.1 million new cases and 1.8 million deaths predicted worldwide in 2018 (2). However, the incidence and mortality rates of LC vary in different geographical areas, as well as between sexes in those areas (3). According to the Iranian National Population Based Cancer Registry (INPCR) in 2014, LC is one of the five most common cancers in Iranian men, but not in Iranian women (4). The lowest incidence in LC in both sexes was observed in Fars province, while the highest was observed in Golestan and Tehran in men and women respectively (5).
According to conventional classification, LCs include non-small cell carcinoma (NSCLC), which accounts for 80% of cases, and small cell carcinoma (SCLC), which accounts for 20% of cases. The major types of NSCLC include adenocarcinoma (ADC), squamous cell carcinoma (SCC), and large cell carcinoma (LCC). Small cell carcinoma is grouped with other tumors that exhibit neuroendocrine differentiation (6).
Numerous risk factors for LC have been reported. In general, these factors include lifestyle, exposure to environmental and occupational factors (such as working in coal mines, textile-related jobs, insulation, construction, automobile repair, shipbuilding, and asbestos that increases oxidative damage), geographical area of residence, gender, racial characteristics, genetic predisposition, smoking (depending on the daily number and annual packs), comorbidities such as chronic obstructive pulmonary disease (COPD), specific diet and genetic factors (7).
Lung cancer is a significant health concern in Iran, with its incidence and histological subtypes exhibiting distinct patterns compared to global trends. A systematic review and meta-analysis published in 2017 reported age-standardized rates (ASRs) of LC at 6.33 per 100,000 for males and 2.57 per 100,000 for females in Iran. These rates are relatively lower compared to many other regions worldwide (5). Regarding the histological subtypes in Iran, SCC is the most prevalent among Iranian men (accounting for approximately 802 cases per 100,000); adenocarcinoma In Iranian women is the most common subtype, with an estimated prevalence of 319 cases per 100,000. The male-to-female ratio for LC prevalence in Iran is approximately 2.01, indicating a higher incidence in men (8).
A study published in 2022 highlighted that cigarette smoking, human papillomavirus (HPV) infection, exposure to mustard gas, occupational hazards, and genetic factors are major reported risk factors for LC in Iran. Conversely, a vegetarian diet has been considered a protective factor (9).
Regarding the prevalence of different cancers in various geographical areas, understanding the incidence of cancer in each region can aid in treating and screening high-risk groups, identifying risk factors, and implementing necessary strategies to eliminate them. There is a varying pattern of LC prevalence across Iran, with different risk factors present in different regions. In Kermanshah, there have been no new findings on the frequency of LC over the past decade.
2. Objectives
This study was conducted to evaluate the clinicopathological characteristics of lung tumors and the survival rate for all types of LC in patients referred to a medical center in western Iran over a ten-year period (2010 - 2019).
3. Methods
All protocols for this study were conducted under the supervision of the review board at Kermanshah University of Medical Sciences (IR.KUMS.REC.1398.288). The reporting of this study adheres to STROBE guidelines. Patients or their legal representatives provided written informed consent prior to the study. Patient details, such as identity, exact age, facial images, and any other identifying information, were not disclosed. Inclusion criteria encompassed all patients with LC (diagnosed via histopathology/computed tomography scan) of any age and gender, residing in western Iran, with accessible clinical information during the study period. Exclusion criteria included patients with additional cancers besides LC, those with LC resulting from metastasis, and those not residing in western Iran or lacking clinical information.
A retrospective study was conducted on LC patients to assess overall survival (OS) rates (time from treatment to death from any cause). Electronic records from a major clinical center in western Iran (Imam Reza Hospital) were utilized from Jan. 1, 2010, to Dec. 31, 2019. Demographic (age, sex, residency status) and pathological data were collected from the clinical files of all 313 patients at Imam Reza Hospital. Additional variables (metastases, survival time, time of death, smoking exposure) were available for respondents to surveys (n = 154). To obtain follow-up and survival information lacking in the clinical record, the listed contact numbers were called.
3.1. Statistical Analysis
The variables under study were described using frequency, percentage, mean, and standard deviation (SD) indices. An independent t-test was used to compare the means of the two independent groups, and Fisher's exact test was employed to determine the relationship between the two variables. The survival rate was estimated using the Kaplan-Meier method, and the log-rank test was applied to compare the survival function. The event in question was specified as death due to LC. Univariable Cox proportional hazards models were utilized to estimate hazard ratios (HRs) for overall mortality. All collected information was analyzed using SPSS software version 22 and Stata 15. A P-value of less than 0.05 indicates statistical significance.
4. Results
During the period of 2010 - 2019 in our study, there were 313 cases related to LC, including 91 (29.1%) females and 222 (70.9%) males (with a male-to-female ratio of 2.42). Of these cases, 235 were in urban areas (75.1%) and 76 in rural areas (24.3%). The mean and median age was 61.17 ± 14.37 and 63 years old, respectively, with the highest frequency occurring in individuals over the age of 50 (84%). There was no significant difference between the mean age of males (61.83 ± 14.38) and females (59.54 ± 14.23) (P = 0.2).
The most common type of tumor recorded was NSCLC (235 cases; 75.1%), followed by SCLC (43 cases; 13.7%), and unclassified tumors (35 cases; 11.2%). The majority of NSCLC cases were in stage I and II (44.7%) and then stage IV (30.1%), while most SCLC cases were in the extensive stage (80%).
Out of the total number of patients studied, information on survival, smoke exposure, and metastasis was available for 154 cases, with 133 cases deceased and 21 surviving. The mean ± SD (95% CI) and median survival were estimated at 22.53 ± 3.17; 16.40 - 28.65) months and 7 months, respectively (Table 1).
| Variables | Deceased | Survived | Total | Survival | 95% CI | P-Value |
|---|---|---|---|---|---|---|
| Gender | 0.239 | |||||
| Male | 93 | 14 (13.1) | 107 (69.5) | 21.68 ± 3.73 | 14.37, 28.98 | |
| Female | 40 | 7 (14.9) | 47 (30.5) | 19.58 ± 3.59 | 12.55, 26.61 | |
| Age group | 0.001 | |||||
| ≥ 50 | 11 | 9 (45) | 20 (13) | 50.45 ± 11.56 | 27.79, 73.13 | |
| < 50 | 122 | 12 (9) | 134 (87) | 17.81 ± 2.81 | 12.30, 23.32 | |
| Residency | 0.355 | |||||
| Urban | 98 | 13 (11.7) | 111 (72) | 20.75 ± 3.46 | 13.98, 27.53 | |
| Rural | 35 | 8 (18.6) | 43 (28) | 24.91 ± 5.94 | 13.26, 36.56 | |
| Smoke exposure | 0.097 | |||||
| Yes | 93 | 12 (11.4) | 105 (68) | 18.36 ± 3.14 | 12.20, 24.53 | |
| No | 39 | 9 (18.8) | 48 (31) | 29.43 ± 6.31 | 17.07, 41.80 | |
| Metastasize | 0.204 | |||||
| Yes | 58 | 5 (7.9) | 63 (42) | 11.70 ± 1.57 | 8.63, 14.78 | |
| No | 74 | 15 (16.9) | 89 (58) | 26.20 ± 4.49 | 17.40, 34.99 | |
| Tumor classification | 0.935 | |||||
| NSCLC (SCC) | 60 | 7 (10.4) | 67 (43.5) | 18.76 ± 3.77 | 11.37, 36.16 | |
| NSCLC (LCC) | 2 | 1 (33.3) | 3 (2) | 16 ± 10.66 | 0.0, 36.89 | |
| NSCLC (ADC) | 35 | 7 (16.7) | 42 (27.3) | 26.42 ± 6.51 | 13.66, 39.18 | |
| SCLC | 18 | 4 (18.2) | 22 (14.3) | 24.09 ± 8.51 | 7.41, 40.77 | |
| Metastatic tumors | 15 | 1 (6.3) | 16 (10.4) | 12.63 ± 3.79 | 5.20, 20.05 | |
| Tumor type | 0.840 | |||||
| NSCLC | 99 | 15 (13.2) | 114 (74) | 22.26 ± 3.55 | 15.30, 29.23 | |
| SCLC | 18 | 4 (18.2) | 22 (14.3) | 24.09 ± 8.51 | 7.41, 40.77 | |
| Metastatic tumors | 15 | 1 (6.3) | 16 (10.4) | 12.63 ± 3.79 | 5.20, 20.05 |
Abbreviations: NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; LCC, large cell carcinoma; ADC, adenocarcinoma; SCC, squamous cell carcinoma.
a Values are expressed as No. (%) or mean ± SD.
Treatment regimens for curable NSCLC cases were cisplatin/vinorelbine (stage I & II ) and cisplatin (stage III); for curable SCLC cases, they were cisplatin/etoposide (stages I - III) and carboplatin/etoposide (stage IV).
There was a statistically significant difference between variables of gender (P < 0.001), age groups (P = 0.012), and smoke exposure (P = 0.005) with tumor classification (Table 1). However, there was no statistically significant difference between variables of metastasizing (P = 0.227), residency (P = 0.954), and survival status (P = 0.537) with tumor classification (Table 2).
| Variables | Frequency of Each Tumor Classification (%) | Other and Unclassified Carcinoma | Metastatic Tumors | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| NSCLC/ SCC | NSCLC/ LCC | NSCLC/ ADC | NSCLC Rare | SCLC | ||||
| Gender | 0.0001 | |||||||
| Male | 107 (81.7) | 7 (87.5) | 59 (63.4) | 1 (33.3) | 36 (83.7) | 1 (100) | 9 (32.1) | |
| Female | 24 (18.3) | 1 (12.5) | 34 (36.6) | 2 (66.7) | 7 (16.3) | 0 (0.0) | 19 (67.9) | |
| Age group | 0.012 | |||||||
| 50 ≥ | 13 (9.9) | 2 (25.0) | 15 (16.1) | 2 (66.7) | 5 (11.6) | 0 (0.0) | 9 (32.1) | |
| 50 < | 118 (90.1) | 6 (75.0) | 78 (83.9) | 1 (33.3) | 38 (88.4) | 1 (100) | 19 (67.9) | |
| Residency | 0.954 | |||||||
| Urban | 96 (73.3) | 6 (75.0) | 73 (78.5) | 2 (66.7) | 31 (73.8) | 1 (100) | 21 (75.0) | |
| Rural | 35 (26.7) | 2 (25.0) | 20 (21.5) | 1 (33.3) | 11 (26.2) | 0 (0.0) | 7 (25.0) | |
| Smoke exposure | 0.005 | |||||||
| Yes | 57 (83.8) | 1 (33.3) | 24 (58.5) | 1 (50.0) | 15 (68.2) | 0 (0.0) | 8 (50.0) | |
| No | 11 (16.2) | 2 (66.7) | 17 (41.5) | 1 (50.0) | 7 (31.8) | 0 (0.0) | 8 (50.0) | |
| Metastasize | 0.227 | |||||||
| Yes | 23 (33.8) | 2 (66.7) | 21 (51.2) | 0 (0.0) | 9 (40.9) | 0 (0.0) | 9 (56.3) | |
| No | 45 (66.2) | 1 (33.3) | 20 (48.8) | 2 (100) | 13 (59.1) | 0 (0.0) | 7 (43.8) | |
| Survival status | 0.537 | |||||||
| Deceased | 60 (89.6) | 2 (66.7) | 35 (83.3) | 2 (100) | 18 (81.8) | 0 (0.0) | 15 (93.8) | |
| Alive | 7 (10.4) | 1 (33.3) | 7 (16.7) | 0 (0.0) | 4 (18.2) | 0 (0.0) | 1 (6.3) | |
Abbreviations: NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; LCC, large cell carcinoma; ADC, adenocarcinoma; SCC, squamous cell carcinoma.
a Values are expressed as No. (%).
The OS rates at 1, 3, and 5 years were 31%, 15%, and 12%, respectively.
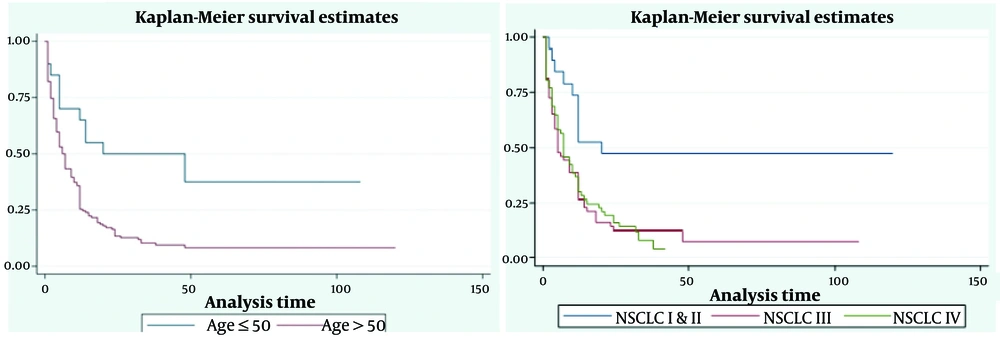
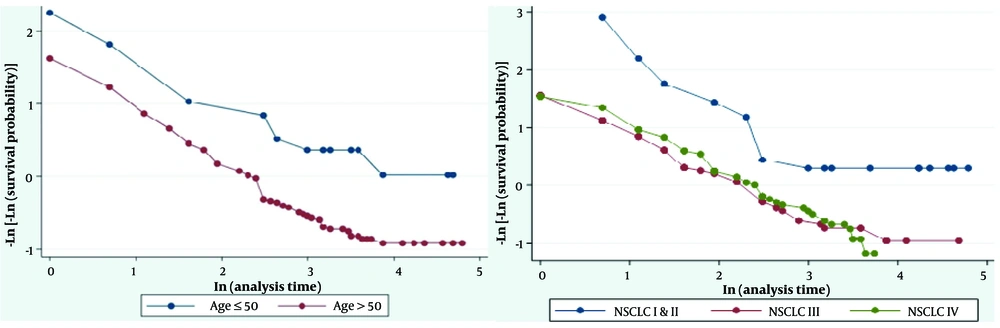
Based on the results of the log-rank test, there was no statistically significant difference between the mean survival of patients by gender (P = 0.239), residency (P = 0.355), smoke exposure (P = 0.097), metastasis (P = 0.204), classification (P = 0.935), SCLC stages (P = 0.375), and tumor type (P = 0.84) (Table 1). However, there was a statistically significant difference between the mean survival in the two groups: Age > 50 years and ≤ 50 years (P = 0.001), and NSCLC stages (P < 0.001) (Table 1). The estimation of survival of LC patients based on age group (months) and NSCLC stage is shown in Figure 1. Additionally, Figure 2 displays the graph of -ln (-ln (S (t))) by age group and NSCLC stage, indicating that the assumption of proportional hazards is violated only for the NSCLC stage.
Furthermore, through the goodness-of-fit test at a significance level of 0.05, it was determined that the proportional hazards assumption was met for the age group (Rho = 0.046; chi-square = 0.29; P = 0.593) but violated for the NSCLC stage (Rho = 0.98; chi-square = 93.45; P < 0.001). Therefore, the stratified Cox model was utilized, and the NSCLC variable was considered a strata. The Cox regression model reveals that patients in the age group > 50 years are 2.7 times more likely to die than those in the age group of < 50 years (HR: 2.034; 95% CI: 1.046 - 3.955, P = 0.036) (Table 3).
| Variable | Coefficient (B) | Standard Error | P-Value | HR (Exp(B)) | 95.0% CI for HR (Exp(B)); Lower-Upper |
|---|---|---|---|---|---|
| Age (0: < 50, 1: ≥ 50) | 0.710 | 0.339 | 0.036 a | 2.034 | 1.046 - 3.955 |
Abbreviation: HR, hazard ratios.
a P < 0.05 was considered statistically significant.
5. Discussion
We evaluated the clinicopathological characteristics of lung tumors and the survival rate of all types of LC in patients in Kermanshah, located in the west of Iran. In our study, the five-year survival rate of LC was found to be similar to results reported in other parts of the world, at less than 15%. We identified the age group of over 50 years old as a risk factor for LC and associated with a higher mortality rate.
Lung cancer has become the most commonly diagnosed cancer globally in recent decades. In 2018, an estimated 2.1 million new cases of LC were diagnosed, accounting for 12% of the global cancer burden. Among men, LC remains the most frequently diagnosed cancer, with approximately 1.37 million new cases in 2018. Among women, the overall incidence is lower than in men, with over 725,000 new cases of LC diagnosed in 2018. Geographical variations in LC incidence between men and women are attributed to historical differences in smoking patterns (10). Despite advancements in cancer treatment, there has been little improvement in the 5-year survival rate of LC patients. This lack of improvement can be attributed to the fact that most patients are diagnosed at a late stage of the disease (11).
Despite diagnostic and therapeutic advances, little progress has been made in the five-year survival rate of LC. In developed countries, the survival rate is less than 20%, while in developing countries, it is about 10%. knowledge of epidemiology and its risk factors in any geographical area can serve as the basis for LC prevention (12). Possible risk factors for LC mentioned in various articles include tobacco smoking, secondhand smoke, electronic cigarettes, other forms of tobacco use (such as cigars, pipes, and water pipes), smoked cannabis, radon exposure, asbestos exposure, a history of COPD, emphysema, or chronic bronchitis, a history of asthma, a history of pneumonia, a history of chlamydia pneumonia, a history of tuberculosis, and HIV (10).
Previous studies have shown that the OS of LC depends on factors such as sex, age, stage of LC (I, II, and III), and histopathologic type. It has been suggested that the simultaneous occurrence of different variables leads to better outcomes in females and those with ADC types (13). Cigarette smoking is the primary risk factor for LC and accounts for the majority of cases in both men and women (7). However, differences in LC incidence between genders may also be influenced by hormonal, genetic, and cultural factors, which can modulate disease susceptibility and progression. According to our results, the frequency of men with LC was 2.4 times higher than women. Male gender has been identified as one of the risk factors for LC in previous studies. Hormonal (12, 13), genetic (14), and cultural (15) differences are factors that can influence the sex-linked incidence of LC. The incidence of LC in men has been decreasing steadily over the past 40 years in the U.S., from 90 per 100,000 in 1975 to 71 per 100,000 in 2010 - 2015, while in women it has been increasing from 25 per 100,000 in 1975 to 52.3 per 100,000 in 2010 - 2015. With advancements in diagnostic and therapeutic methods for LC, a decrease in its incidence has been observed in the last decade. This decrease was 3% per year for men between 2011 and 2015, while 1.5% per year was reported for women (11). In other words, the reduction in the incidence of LC in women has a slower rate than in men. The most recent literature on LC in the Middle East and North Africa (MENA) is summarized in Table 4.
| Authors (Year of Publication) | Outcome |
|---|---|
| Salim et al. (16) | They investigated the incidence rate of LC and the mortality rate of Arab countries and reported that there is a great variation in the incidence rate of LC in this region. The ASR for LC in Tunisia is 15 times higher than in Sudan in men and in Bahrain is 10 times higher than in Yemen in women. With the exception of Algeria and Tunisia, where SCC is the most common type of LC, ADC is predominant in women in other regions. Smoking is the most important risk factor for LC in these areas. |
| Khazaei et al. (17) | In Iran, the most common histological types of LC in men and women are SCC and ADC. The incidence rate of LC is higher in warm provinces of Iran. Climatic conditions, environmental pollution, lifestyle, socio-economic and industrial conditions of the region played a role in the high rate of LC in these regions. |
| Jazieh et al. (18) | Age-standardized rate in the MENA region (including Saudi Arabia, United Arab Emirates of Oman, Qatar, Kuwait, Bahrain, Yemen, Iraq, Syria, Lebanon, Jordan, Libya, Tunisia, Algeria, Morocco and Egypt) is lower than the international rate and its range was between 2.4 per 100,000 in Yemen and 23 per 100,000 in Lebanon. The estimated number of new cases of LC in 2018 was 79,887 and the 5-year survival rate was 8%. The highest percentage of deaths was in Morocco and Tunisia and the lowest in Yemen and Egypt. |
| Khanmohammadi et al. (19) | They estimated the regional and national burden of TBL cancer and its attributable risk factors from 1990 to 2019 in MENA. They reported 15,396 deaths due to TBL in women and 57,114 in men. All the standardized age indices showed a decreasing trend in men and an increasing trend in women. The highest and lowest absolute slopes of change in the Standardized Age Index from 1900 to 2019 were observed in Bahrain and the United Arab Emirates. Tobacco use was listed as the main risk factor. |
| Globocan (20) | The highest percentage of new cases of LC in MEA was reported in Tunisia (15%) and the lowest was in Oman (3.7%), which included the highest and lowest of deaths. |
| Arafa et al. (21) | The reason for the increase in LC rates in the Persian Gulf nationals and the diagnosis of LC in the final stages due to geographical barriers that make it difficult to access care cited the lack of medical infrastructure and trained specialists to provide quality care. |
| Jazieh et al. (22) | The success rate of the pathological diagnosis of LC in patients in Saudi Arabia, the United Arab Emirates, Qatar, Lebanon, and Algeria by histopathologic examination in the first step was 72.3%. In the rest of the patients, other diagnoses, such as image-guided biopsy, surgical biopsy, endobronchial biopsy, and cytology were needed; among all mentioned cases the surgical biopsy and guided biopsy were more successful. |
| Mansour et al. (23) | According to data published in 1997 - 2022, predisposed factors attributable to cancer in MENA include tobacco use, obesity, physical inactivity, and diet. Among these risk factors, tobacco use is the main risk of LC. |
Abbreviations: SCC, small cell carcinoma; ASR, age-standardized rate; ADC, adenocarcinoma; LC, lung cancer; MENA, Middle East and North Africa; TBL, trachea, bronchus, and lung.
Based on the conventional classification, LC includes NSCLC, SCLC, and metastatic tumors. Non-small cell carcinoma comprises four subtypes: Adenocarcinoma, SCC, LCC, and rare NSCLC. Adenocarcinoma, once known as "Bronchoalveolar carcinoma", can disproportionately affect women. It has been observed that patients with this subtype have a longer survival time than other subtypes, but their intrathoracic recurrence is higher than other NSCLC subtypes (24). On the other hand, the most common subtype of LC in men is SCC (15). In the present study, in line with the mentioned findings, the OS rate was higher in ADC (26.42%), and it was the most prevalent morphology in women (36.6%). This subtype was also more common in people who were not exposed to smoke. Conversely, the most common subtype in men who were exposed to smoke was SCC. Overall, the most typical type of LC morphology in our study was SCC. This result contradicted new findings suggesting that the prevalence of SCC has been declining since the 1990s (25) and that the incidence rate of ADC in men and women is rising globally (26, 27). According to the risk factors for SCC of the lung, which include smoking, age, family history, exposure to second-hand smoke, minerals and metal particles, or asbestos, the lifestyle and habits of people in the region, particularly excessive tobacco use, are likely reasons for the increase in this type of LC (28).
In this study, the proportion of urban residents was higher than that of rural residents. However, residence status did not affect survival time. Factors contributing to the higher frequency of LC among urban patients include air pollution (29) and smoking culture (30). One reason for the prevalence of LC in non-smokers is changes in particulate matter, which can impact the incidence of ADC and patient survival (29). A retrospective study using cancer databases found that living in a rural area is an independent risk factor for reduced survival in all stages of NSCLC, particularly in stage I. Lack of access to medical facilities and diagnostic equipment in rural areas is a significant factor in this disparity. Additionally, rural health centers lack the same level of coordinated instructions as urban health centers (31).
Most of the patients in the present study were over 50 years old. It has been suggested that cancer is related to aging. Therefore, in addition to smoking and other risk factors, the rising elderly population is another risk factor for LC (32). Lung cancer has also been shown to be more aggressive in younger patients under 50 than in older patients (33). The median progression-free survival (PFS) has an interesting pattern across age groups, with previous studies reporting that the median PFS is 1.81 months for those under 60 years, 2.53 months for those 60 - 69 years, 3.75 months for those 70 - 79 years, and 1.64 months for those 80 years and above. In other words, the age group 70 - 79 years had a significantly lower hazard for disease progression or death than younger patients (34).
Smoking is a negative independent prognostic factor for LC. The duration and frequency of smoking can affect the histopathologic type diagnosis of LC. The most common histopathologic subtypes induced by smoking are SCC, ADC, and SCLC (32). In the diagnosis of LCC and ADC, the duration of smoking is important, and in the diagnosis of SCC, the number of times smoking (35). The duration of smoking is important in diagnosing LCC and ADC, while the number of cigarettes smoked is important in diagnosing SCC. Previous studies have suggested that LC in nonsmokers differs clinically from tobacco-related LC, potentially due to genetic or pathogenic factors (36). Nonsmokers typically present at a later stage of the disease, with stage IV being more common. Studies have shown that exposure to lung carcinogens like asbestos, arsenic, radon, cadmium, nickel, metal dust (37), particulate matter (29), and fumes from fried oil can predispose individuals to LC (38). One traditional occupation in Iran involves baking bread with firewood, which has been shown to have similar effects on the expression of proteins involved in LC as tobacco smoke (39). In our study, smoke exposure referred to smoke from burning wood, cigarette smoke in smokers, and second-hand smoke. Non-small cell carcinoma was more common in those exposed to smoke, but exposure did not affect mean survival or act as a risk factor. Despite medical advancements, the five-year survival rate in our study was less than 15%, indicating a lack of improvement in survival for this group of patients.
This study presents the latest epidemiological and histopathological findings, as well as the survival rates of LC patients in Kermanshah, located in western Iran. However, like many epidemiological studies, it encountered limitations such as the incomplete access to patient records and the cross-sectional design of the study. Data was collected within a specified timeframe based on the information available from patients or their relatives. One limitation of this study was the lack of power analysis for calculating the sample size. One of the limitations of our study is that data on survival and exposure to cigarette smoke were only available for 154 patients, which may bias the results and affect the generalizability of the results. One of the limitations of this study is the lack of access to precise data regarding the duration of patient follow-up. Due to this limitation, the follow-up period was not included in the study. This factor may impact the generalizability of the results, and it is important to note that future studies should address this aspect.
5.1. Conclusions
In our study, the five-year survival rate of LC was found to be similar to the results of previous reports from other parts of the world, which is less than 15%. We identified the age group over 50 years old as a risk factor for LC and associated with a higher mortality rate. Various risk factors for LC have been reported, including genetics, occupation, age, air pollution, special diet, smoking, radon exposure, marijuana smoking, alcohol consumption, and infection with HPV, HIV, and Epstein-Barr virus. In different geographical areas, influenced by the culture and customs of the region, each of these risk factors can be considered a significant contributing factor.