1. Background
Methylphenidate hydrochloride (MPH, Ritalin®) is a psychostimulant that has a similar structure to dextroamphetamine sulfate, a kind of amphetamine isomer (1). Methylphenidate hydrochloride is used to treat attention-deficit/hyperactivity disorder (ADHD) in children and adults as a first-choice drug (2). Methylphenidate hydrochloride has a high potential for abuse among adults due to its striking resemblance to some addictive drugs (3).
Methylphenidate hydrochloride inhibits the presynaptic reuptake of dopamine and norepinephrine, resulting in a rise in their concentration, particularly in the hippocampus and prefrontal cortex, which are critical for memory regulation and synaptogenesis (4) Learning increases synaptic activity in the hippocampus-prefrontal cortex pathway (5). After increasing the amount of dopamine, the motor inhibitory system in the orbital frontal axis is activated. Hence, the focus of attention has been improved (6). Despite this, there are still results that show an obvious inconsistency in the effect of MPH on learning and memory (7). There is ample evidence to demonstrate deficits in task performance of laboratory animals treated with MPH (8-11). On the other hand, some reports show an improvement in memory or cognitive behavior following the administration of MPH (12). The hippocampal formation undeniably plays an indispensable role in spatial memory and learning (13), and is also involved in other types of memory (14). This theory is reliant upon research in which the hippocampus in laboratory animals is removed (15) or damaged (16, 17), showing impairment in the performance of tasks related to memory and/or learning. Also, the hippocampal-prefrontal cortex pathway has a critical role in memory consolidation (5). Dentate gyrus (DG) as a hippocampal region play a vital role in the spatial learning (18).
As mentioned, the administration of MPH leads to an increase in the level of DA as well as NE in various brain regions, including hippocampal structures (12). These increases can lead to various consequences, including neurotoxic effects (19, 20). The toxicity of dopamine has been demonstrated in cultured cells (20-22) and there have been reports of neuron loss (23).
Numerous studies have investigated the long-term effects of MPH on memory and motor function, yielding inconsistent results (8, 11, 24-26). It is crucial to examine the effects of the drug after both single-dose and repeated administration within single research in order to separate the effects from their dose dependency. Nevertheless, no study has concurrently examined the drug's short-term and long-term effects at clinical and high dosages.
2. Objectives
Due to inconsistencies in previous investigations on the effects of MPH on memory, and the lack of studies exploring the relationship between the histopathological changes in the cornu ammonis (CA1) and DG regions of the hippocampus and spatial memory, this study aims to examine the short-term and long-term effects of MPH as well as how different doses of the drug affect spatial memory in rats and to investigate the possible connection between hippocampal neuron degeneration and memory performance.
3. Methods
3.1. Animals
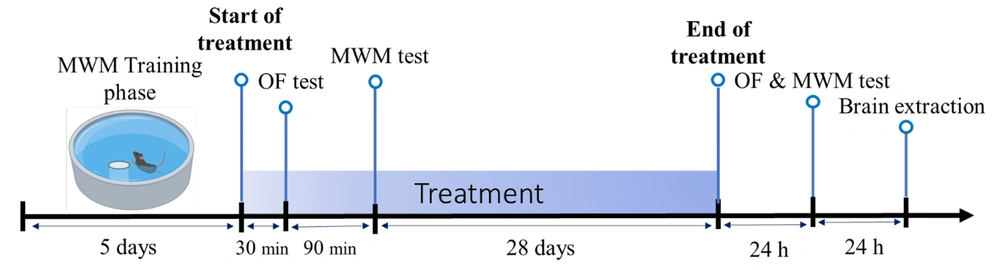
A total of 40 adult male albino Wistar rats were purchased and maintained under a 12-h light/dark cycle (lights on 07:00 - 19:00 h) and a temperature of 22 ± 2°C in plastic cages (5 animals per cage) for acclimatization within seven days. Rats weighed 250 – 335 g at the beginning of the study. A Standard food diet was available as well as water and rats were equally and randomly categorized into four groups; three experimental groups and one control groups.
3.2. Drug
Methylphenidate hydrochloride (Ritalin, Novartis Pharma) tablets were dissolved in saline just before each administration for experimental groups.
3.3. Experimental Design
Animals underwent 28 days of fresh solution MPH (0.6, 2.5, and 10 mg/kg) or saline (control) orally by gavage once a day. Meanwhile, all administrations were between 11:00 and 12:00 a.m. and the doses of MPH were selected based on a previous study, in which these three doses were used to investigate a dose-response effect (27). In this investigation, low doses of 0.6 and 2.5 mg/kg were determined based on clinical relevance for humans, whereas high doses of 10 mg/kg were determined based on the possibility that they could be abused and result in overdoses.
3.4. Apparatus
3.4.1 Open Field Test
The behavioral procedures started in a square wooden box (60 × 60 cm) with 40 cm high walls and the floor was equally divided by white lines into 16 squares, as described formerly (10). To evaluate the impact of a single dose of MPH on motor activity, animals were tested 30 minutes after the initial administration based on pharmacokinetic properties of the drug in rat (11, 26). The animals were gently placed into one of the corner squares of the apparatus, and the frequency of squares crossed was recorded for a period of 5 minutes. Subsequently, MPH or saline was administered daily for a total of 28 days. To assess the effects of long-term administration, the same open field task was repeated 24 hours after the final administration of MPH. This allowed for the evaluation of motor activity following both the acute and chronic administration of MPH.
3.4.2. Morris Water Maze
The Morris water maze (MWM) is widely recognized as one of the most important tools for assessing the negative impacts on the hippocampus and evaluating spatial memory in laboratory animals (28). The procedure of the MWM test was designed to estimate spatial learning and memory based on a previous study (29). The task included five days of training phase and one day of test sessions. On each day of the training phase, rats got four trials per day and the interval between trials was ten minutes. In every trial, a rat was placed in the water facing the tank wall, in one of the four distal starting positions (NE, N, W, and SW) and was given one minute to navigate the submerged escape platform that was set within the SE quadrant, 2 cm underneath water. It is worth noting that the order of starting positions changes on a daily basis. Animals that found the hidden platform were allowed to stay on the platform for 15 seconds to learn to how find a direct path to the platform using the cues, while those that did not find the platform were directed to the platform and looked for 15 seconds for a similar purpose. Instantly after the training, the platform was picked, and the animals received either a single dose of MPH or saline. Test sessions began 2 hours after the last training phase, to assess the single-dose effect of the drug on memory. In all test sessions, the latency to find an escape platform was monitored and the starter location was in the north-west (NW) where the animals had not been placed throughout the training phase. To evaluate the effects of long-term administration on memory, the animals received daily doses of MPH and were tested after four weeks.
3.5. Section Preparation
In addition to behavioral tests, histopathologic observations were conducted to investigate the potential neurodegenerative effects of long-term MPH treatment. Twenty-four hours after the behavioral tests, the animals were deeply anesthetized with a ketamine cocktail (ketamine: 50 mg/kg, xylazine: 5 mg/kg. Following this, seven brain samples from each group were extracted. Subsequently, the hippocampal tissue was removed, and 0.5 cm-thick tissue slices were prepared and preserved in a 10% formaldehyde solution. Tissue sections of 5 µm thickness were provided from the samples, using a paraffin technique. They were stained by applying the Hematoxylin and Eosin (H&E) staining procedure. Further, they were photographed using a photomicroscope. All the tissue alterations were compared to the control group and recorded via the obtained images. In the microscopic examination, the number of degenerated neurons, as well as the number of intact neurons in the CA1 and DG regions were counted by the ImageJ Software, and the ratio of degenerated neuronal cells in the hippocampal regions (% degenerated neurons/total number of neurons) was analyzed. These histopathologic analyses aimed to determine the extent of neuron degeneration. Figure 1 shows a timeline of the whole experimental procedures in this study.
3.6. Statistical Analysis
The data were analyzed using GraphPad Prism 10 software, and the significant level was considered P < 0.05. One-way analysis of variance (ANOVA) followed by Tukey’s test was performed to determine the differences between groups in the Morris water maze, open field and neurodegeneration rate.
4. Results
4.1. Locomotor Activity in Open Field
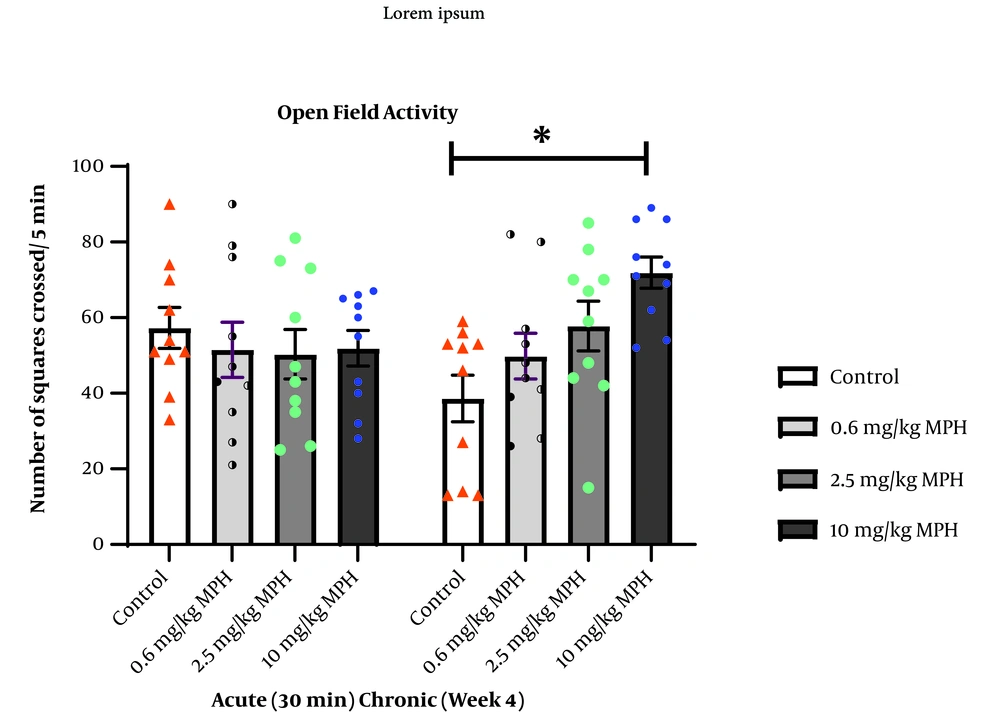
Figure 2 illustrates the dose-dependent effects of a single dose of MPH on open field activity. One-way ANOVA indicated that none of the doses revealed any significant difference (F3,36 = 0.2620, P = 0.8523). However, One-way ANOVA showed a significant difference between groups in locomotor activity in the open field following long-term administration of MPH (F3,36 = 5.819, P = 0.0024), with Tukey’s test showing an increase in the frequency of squares crossed in rats treated with a high dose of MPH compared to the control group (P = 0.0014).
Dose-dependent effects of short-term (acute) and long-term (chronic) use of methylphenidate hydrochloride (MPH) on motor activity in the open field. Values are means ± SEM (n = 10 rats per group). * P < 0.05 versus all other groups; calculated by one-way analysis of variance (ANOVA) with Tukey's post hoc test.
4.2. Spatial Memory in Morris Water Maze
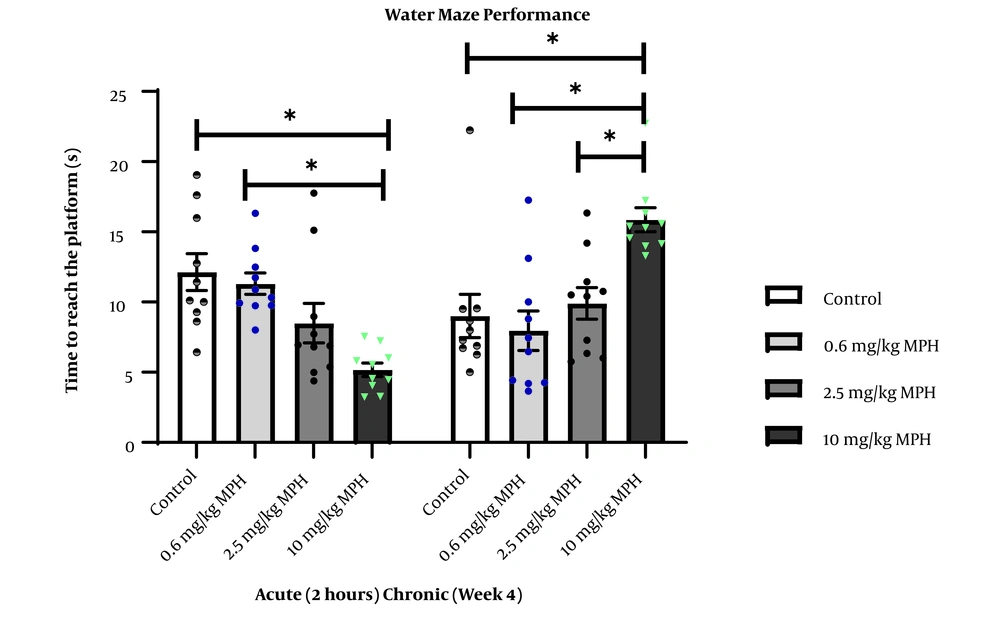
A decrease in the latency to reach the hidden platform is described as memory improvement, whereas an increase in the latency to reach the platform is defined as memory impairment. The effects of single and long-term MPH treatment on the memory of rats are illustrated in Figure 3. One-way ANOVA showed a significant difference between groups in the latency time following a single dose of MPH (F3,36 = 8.776, P = 0.0002), with Tukey’s test showing a decrease the latency time of rats treated with 10 mg/kg of MPH compared to control (P = 0.0003) and rats treated with 0.6 mg/kg of MPH (P = 0.0013). After four weeks of repeated administration, animals exposed to the same maze. One-way ANOVA exhibited a significant difference in the delay in finding the hidden platform (F3,36 = 7.906). Follow-up Tukey’s test showed an increase in the latency time of rats treated with long-term use of 10 mg/kg MPH compared to the control group (P = 0.0025), and rats treated with 0.6 mg/kg (P = 0.0005) and 2.5 mg/kg of MPH (P = 0.01).
4.3. Histopathologic Observations
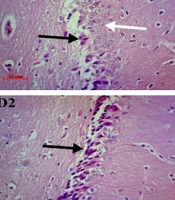
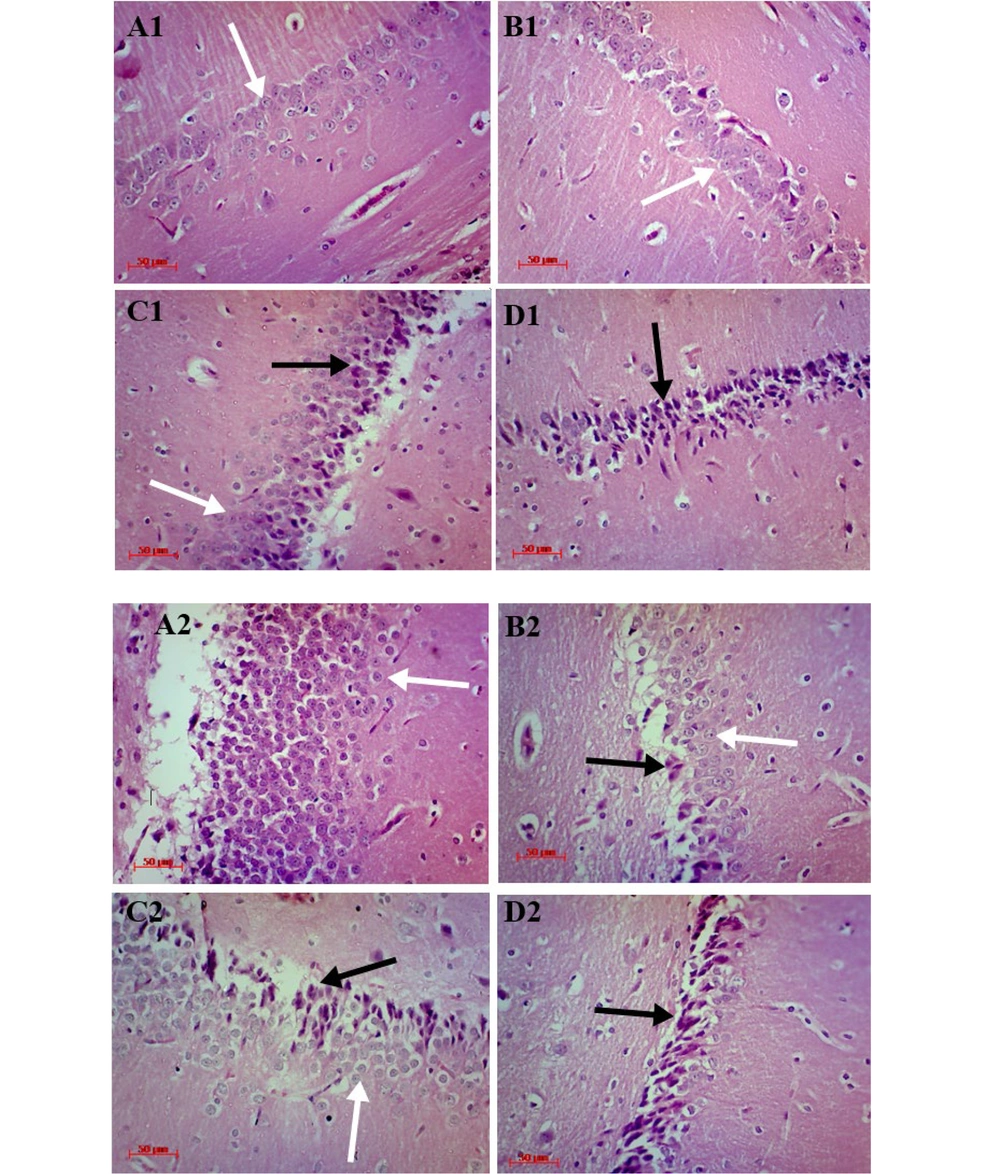
In both control groups, most neurons of the hippocampal formation had light cytoplasm, euchromatin, spherical nucleus and central nucleolus, while in the MPH-treated groups, most of the pyramidal neurons in CA1 and granular neurons in DG were changed to an abnormal cell (triangular) with eosinophilic and condensate cytoplasm, hyperchromatic nucleus, and an absence of nucleolus. These alterations were dependent on the dosage (Figure 4).
Hematoxylin and Eosin (H&E) staining, exhibits cornu ammonis (CA1) pyramidal cell layer of the hippocampus in rats treated with 0, 0.6, 2.5 and 10 mg/kg methylphenidate hydrochloride (MPH), which shows by A1, B1, C1, and D1 respectively. A2, B2, C2, and D2 show the dentate gyrus (DG) granular layer of the hippocampus in Rats treated with 0, 0.6, 2.5, and 10 mg/kg MPH respectively. Degenerated and intact neurons marked by black and white arrows (↑) respectively (magnification × 400. Scale bar 50 µm).
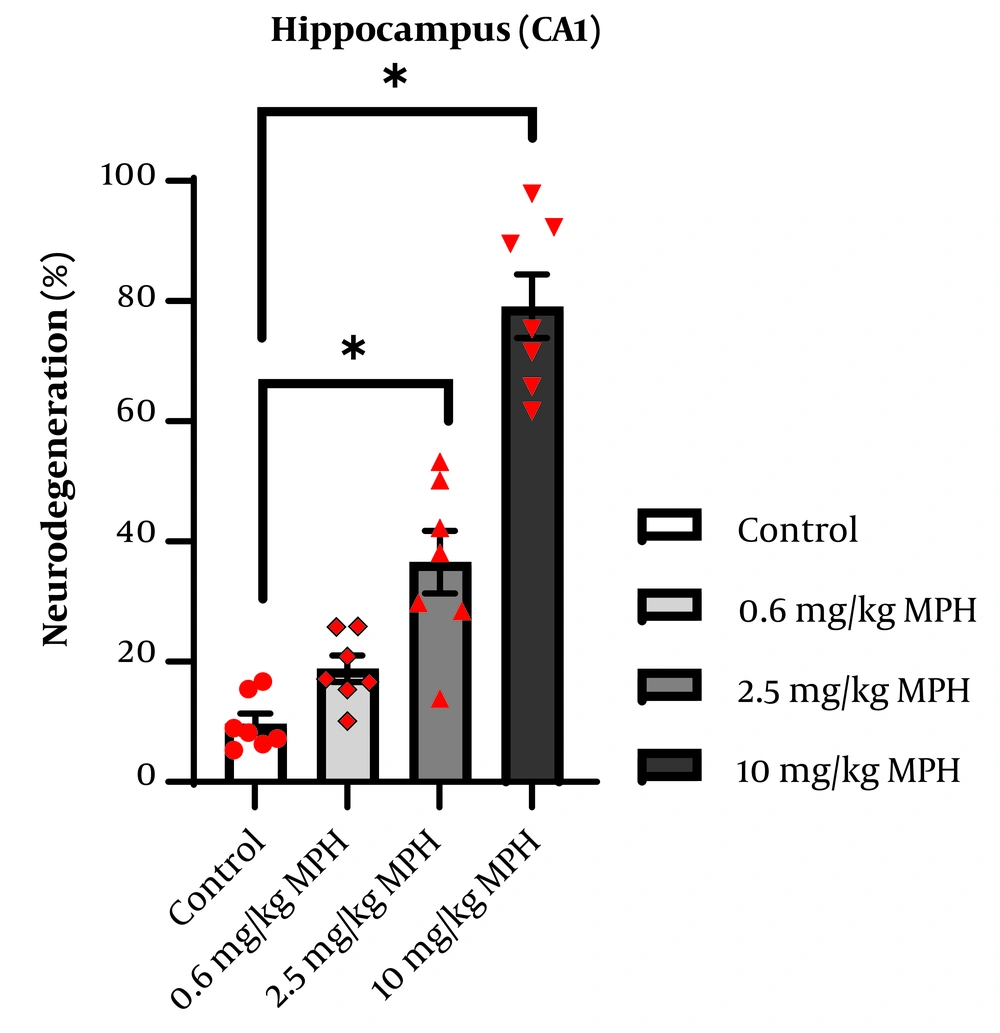
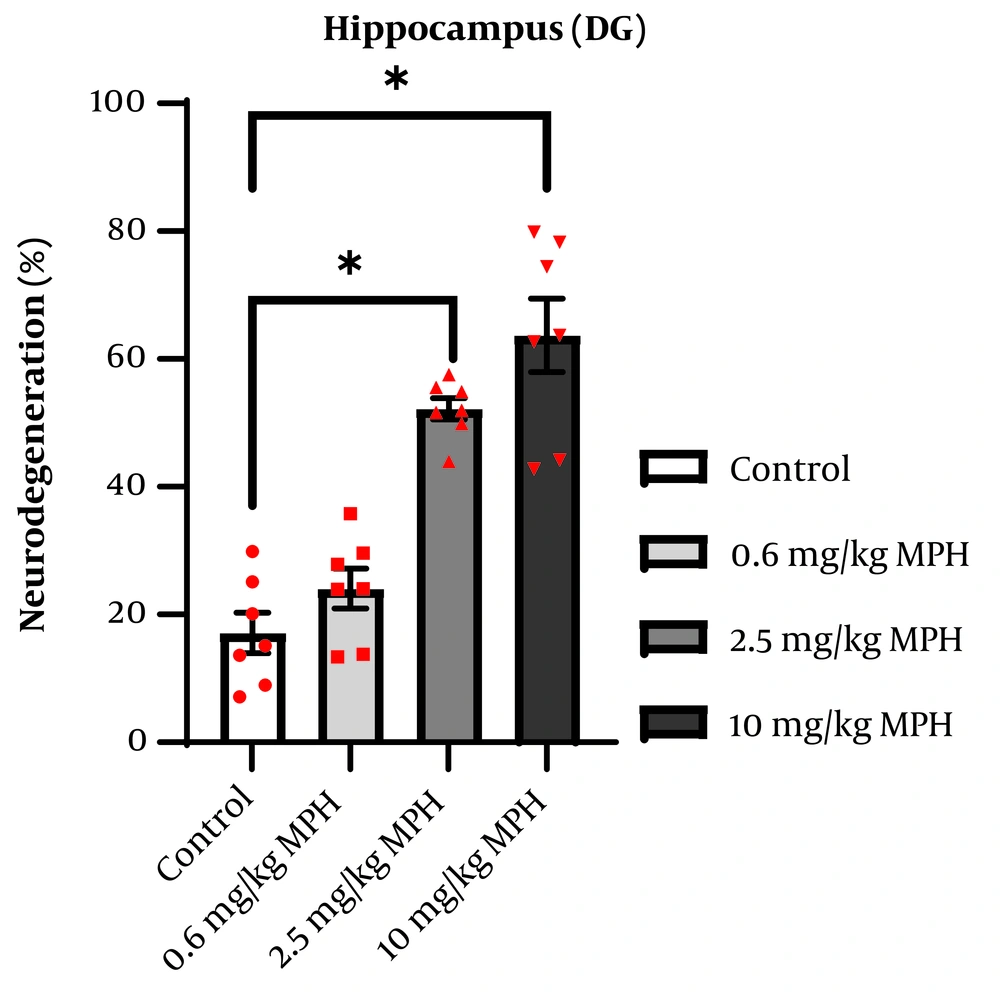
The hippocampus of four mice from each group was inspected, and the ratio of degenerated neurons to total neuronal cells was computed. The average of each group was considered for analysis. The degeneration of pyramidal neurons in the CA1 region is presented as a percentage in Figure 5. One-way ANOVA indicated a significant difference between the groups (F3,24 = 60.49, P < 0.0001). Tukey's test demonstrated that, compared to the control, administering MPH at doses of 2.5 mg/kg (P = 0.0004) and 10 mg/kg (P < 0.0001) significantly raised the percentage of neurodegeneration group in the CA1 region. The degeneration of granular neurons in the DG region is presented as a percentage in Figure 6. One-way ANOVA indicated a significant difference between the groups (F3,24 = 35.38, P < 0.0001). Follow-up Tukey’s test showed that MPH at doses of 2.5 mg/kg (P < 0.0001) and 10 mg/kg (P < 0.0001) significantly raised the percentage of neurodegeneration in the CA1 region compared to the control group.
5. Discussion
In the present study, there was no deficiency in the locomotor activity of animals as revealed by the OF test. The motor activity following long-term use of MPH increased in a dose-dependent manner, specifically at high dose, which in ADHD patients might be considered problematic for the worsening of hyperactivity. Long-term administration of 1 mg/kg MPH induced behavioral sensitization in Wistar rats. This may represent impulsivity in ADHD patients (25). Also, it has been reported that chronic use of 2.5 mg/kg MPH produces behavioral sensitization through the glutaminergic system in the caudate nucleus of Sprague-Dawley rats (30).
The elevation of catecholamines like dopamine and norepinephrine in different brain regions, which rises after Ritalin use, is one of the primary causes of increased motor activity (25). In addition to DAT inhibition, other mechanisms also contribute to dopamine control, which in turn affects motor activity, as studies indicate that the 5-HT1A receptor probably plays a role in dopamine regulation (31). High doses of Ritalin have been shown by Zhang et al. to alter NMDA receptors in the prefrontal cortex, which leads to an increase in motor activity (32).
In addition, the present study demonstrates that a single dose of MPH significantly improves memory at high dose (10 mg/kg) in rats. However, long-term administration of MPH impairs memory at high dose. In support of this data, it has been shown that long-term use of intraperitoneal administration of 2 mg/kg MPH per day impairs the performance of juvenile rats in MWM (8). Oral administration at a dose of 10 mg/kg MPH may lead to the processes of memory formation impairment except memory retrieval in adult rats as shown by object recognition test (33). Furthermore, 10 mg/kg intraperitoneal MPH enhances memory acquisition and retention in mice (24). In contrast, MPH at doses of 0.25, 0.5, and 1 mg/kg given by gavage improved all processes of memory, including acquisition, retention, and reconsolidation in adult rats (25). A study conducted by Salman et al. reaches different conclusions, finding that low doses (0.5 and 2.5 mg/kg) of MPH enhance learning and memory, while a high dose (5 mg/kg) of MPH impairs memory (34). According to another study that used the Y-maze to test the rats' memory, a 5 mg/kg dose of MPH had no discernible impact on short-term spatial working memory. Additionally, the study indicated that MPH reduces long-term potentiation (LTP) (35).
One study reported that repeated intraperitoneal administration of 1 mg/kg MPH to rats impairs spatial learning and memory. It revealed that this impairment is due to a decrease in protein kinase A activity–an enzyme involved in the translocation of the Glu1 subunit of the AMPA receptor–in the hippocampus, which subsequently leads to reduced AMPA receptor trafficking and LTP, thereby causing dysregulation of synaptic plasticity (36).
We also found that high dosages of MPH over time lead to neurodegeneration in the hippocampus areas (CA1 and DG). This neurodegeneration may be linked to the memory impairment observed in the MWM performance and provide an explanation for why repeated administration of MPH at high dose causes brain damage while a single high dose may enhance memory by raising neurotransmitter levels in synaptic clefts. In support to these findings, it has been proposed that memory enhancement may be attributed to an increase in neurotransmitters such as noradrenaline and dopamine, while memory impairment may arise from the serotoninergic effects of high doses of MPH (34).
Hippocampal regions are involved in memory and learning. For example, DG has a vital role in short-term memory (17) and the connection between the hippocampus and PFC is critical for memory consolidation (5). Cornu ammonis is one of the most susceptible regions of the hippocampus to ischemic stroke (37). In our study, we hypothesized that the observed memory impairment due to long-term administration could be a result of neuron degeneration, particularly in high dose.
Chronic exposure to stimulants has a great impact on various cells in different brain areas. For instance, MPH has devastating effects on the activity of pyramidal neurons in the PFC of juvenile rats (38). Consistent with our investigation, the study by Meftahi et al. found that after receiving 5 mg/kg of MPH, the hippocampal astrocyte count increased, potentially causing inflammation and cell death (35). In line with our present study, Schmitz et al., using 2 mg/kg MPH i.p. once a day (for 28 days), indicated that impairment in the object recognition task may be due to a reduction in the number of hippocampal neurons and astrocytes (23). In another study, Coelho-Santos et al. demonstrated that chronic use of 5 mg/kg MPH might impair cognition and memory by damaging the blood-brain barrier in the hippocampus, which results in memory impairment (39). Another research conducted by Andreazza et al. demonstrates that persistent treatment with MPH, particularly at a dose of 10 mg/kg, causes DNA damage in young and adult rats (40).
All in all, there are a few explanations regarding the mechanisms for the devastating effects of MPH, including neuron loss and neurodegeneration in the brain. One explanation may be the decline in the level of hippocampal tropomyosin receptor kinase B (TrkB) and vascular endothelial growth factor (VEGF) following chronic administration of MPH in mice; lack of these factors can lead to a deficit in hippocampal neurogenesis and cell survival (41). Another rational explanation for neurodegeneration is the augmentation of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin one beta (IL-1β) (42) or interleukin six (IL-6) (23) subsequent to chronic MPH exposure in the CA1 and DG regions of the rat’s brain. Stress oxidation can be counted as another explanation in which expansion of monoamines after exposure to some psychostimulants causes the production of reactive oxygen species, thereby causing neuroinflammation and cell death in many brain regions, including the hippocampus, in a dose, age, and administration time-dependent manner (42-44). Another explanation that has been provided by Gopal et al. is to affect the GABA receptor by a high dose of MPH can have devastating impacts on the brain tissue (45). It has also been reported that a clinical dose of MPH causes neurodegeneration via upregulating expression of genes responsible for pro-inflammatory responses (46). Furthermore, long-term administration of 10 mg/kg MPH inhibits cAMP response element-binding protein (CREB), a transcription factor that stimulates the synthesis of neurotrophic factors like brain-derived neurotrophic factor (BDNF) (47). In the central nervous system, the neurotrophin BDNF is essential for the survival and differentiation of neurons (48).
In this study, although we did not evaluate sex as a variable, there is a wide range of factors that could account for sex differences in other studies. One of them is the disparate density of DAT binding sites in diverse brain areas, which is reported as having more binding sites in the female rat striatum than in their male counterparts (49). Another explanation for the sex differences in other investigations could be pharmacokinetic properties. For instance, a study using Sprague-Dawley rats and 5 mg/kg MPH discovered that female rats had greater MPH brain concentrations than male rats, which may be related to lower drug clearance in females (50), while in human subjects, treatment of 0.3 mg/kg MPH resulted in a higher plasma concentration in men than in women (51). Thus, further studies need to investigate potential sex differences in the behavioral and neurobiological responses to MPH, as most existing research has primarily focused on male animals. It is also highly recommended that the recent study be conducted on laboratory models with phenotypes similar to ADHD in humans, such as the spontaneously hypertensive rat.
5.1. Conclusions
Our research findings reveal dose-dependent memory deficits associated with long-term administration of high doses of MPH therapy. These deficits are likely linked to neuronal degeneration in the CA1 and DG regions of the hippocampus.