1. Background
The World Health Organization acknowledges leishmaniasis as a major concern for public health. Amongst leishmaniasis forms, cutaneous leishmaniasis (CL) is the most commonly encountered one. The disease is transmitted through the bite of infected female sandflies and results in chronic skin lesions, which often leave permanent scars. The disease is widespread in many regions, such as Iran, and is mainly attributed to Leishmania major and Leishmania tropica (1-4). The disease has been reported in tropical and semi-tropical areas around the world; however, 90% of the cases are repeated in Afghanistan, Brazil, Iran, Peru, Saudi Arabia, and Syria (5). Considering the important socioeconomic effects of Leishmania as a topical infection, many attempts have been made to develop new vaccines and drugs against it (6). The treatment of leishmaniasis is a great challenge. Existing treatments, including pentavalent antimonials, amphotericin B, pentamidine, and paromomycin, are associated with side effects, high costs, and increasing resistance (5). Consequently, researchers are exploring plant-based compounds as alternative therapies. In the realm of drug development, mitochondria are recognized as important pharmacological targets due to their critical role in energy control, which, when disrupted, leads to irreversible cell damage (7, 8). Also, the enzyme arginase, the first enzyme of the polyamine biosynthetic pathway in Leishmania, has emerged as a potential therapeutic target (9).
The pomegranate (Punica granatum), a deciduous shrub in the Lythraceae family, grows in Mediterranean and tropical regions, reaching 2 to 5 meters (10). It contains anthocyanins, tannins, and phenols. The juice’s red color is due to anthocyanins such as delphinidin, cyanidin, and pelargonidin glycosides. The peel is rich in polyphenols, condensed tannins, catechins, and prodelphinidins (11). Punica granatum extract shows activity against various parasites, including Cryptosporidium, Giardia, Entamoeba, Trichomonas, Plasmodium, nematodes, cestodes, and trematodes (12-18). In addition, a study showed that the hydroalcoholic extract of the flower and fruit peel of P. granatum could inhibit L. major promastigotes due to the presence of compounds such as ellagic acid with antioxidant properties and punicalagin with antimicrobial properties (19). In another study, it was shown that oral treatment with P. granatum juice significantly reduced the average size of CL lesions compared to control mice, with the anti-leishmanial activity being associated with increased antioxidant enzyme activity (20).
2. Objectives
The present study investigates the efficacy of an aqueous extract of P. granatum peel against L. major in both in vitro and in vivo conditions.
3. Methods
3.1. Preparation of Aqueous Extract
The pomegranate peel was purchased from a market in Kermanshah province, Iran. First, an amount of pomegranate peel, free from any dust, was ground into powder using an electric grinder. For aqueous extraction, 100 grams of this powder was immersed in 1000 milliliters of boiling water, and placed on a heater with a magnet for one hour. The resulting mixture was cooled to room temperature, filtered, and then centrifuged. The resulting solvent was concentrated by evaporation at 40°C with the aid of a rotary evaporator.
3.2. Anti-promastigote Assay (In vitro)
This study, as a randomized and controlled trial, was conducted on L. major (MRHO/IR/75/ER) prepared at the Pasteur Institute (Tehran, Iran). The parasite was cultured in NovyMacNeal-Nicolle medium before being mass produced in RPMI 1640 medium containing 10% fetal bovine serum and L-Glutamine, as well as 100 IU/mL penicillin and 100 mg/mL streptomycin for bacterial decontamination (all chemicals obtained from Sigma). After 72 h, the culture medium was centrifuged, and the number of parasites was counted using a Neubauer chamber. Fifty microliters of medium containing promastigotes were added to each well of 24-well plates. Concentrations of 0.1 - 0.9 mg/mL pomegranate extract were prepared, 50 μL of each dilution was added to the wells of the plate, and physiological serum was placed next to them as a negative control and incubated at 25°C for 72 h. Then, 25 μL from each test and control well was added to 25 μL of 0.4% trypan blue, and the number of live and dead parasites was counted with a Neubauer chamber. Each assay was performed in triplicate.
3.3. In vivo Study
Forty healthy adult female BALB/c mice, aged 6 - 8 weeks and weighing between 40 - 50 g, were selected and maintained under standard laboratory conditions (12 h light/dark cycle, 23 ± 2°C, with free access to food and water). Mice were subcutaneously injected at the base of the tail with 2 × 106 L. major promastigotes. Lesions developed within 2 weeks post-infection, and only mice with visible, measurable lesions were included in the treatment phase. Mice were randomly assigned to five groups (n = 8 per group) in a parallel-group randomized controlled trial with an allocation ratio of 1:1:1:1:1. Treatment groups received topical applications of aqueous pomegranate peel extract at concentrations of 2.5%, 5%, 20%, or 40%. The extract was applied using a sterile cotton swab directly to the lesion area, twice daily (every 12 hours) for 30 consecutive days. The control group received physiological serum using the same method and schedule (topical application to the lesion area twice daily for 30 days). All applications were done by the same researcher to ensure consistency. Lesion sizes were measured with a digital caliper at baseline (before treatment) and after the 30-day treatment period to evaluate treatment efficacy.
3.4. Statistical Analysis
Data analysis was conducted using SPSS version 16.0, employing paired t-tests to compare lesion sizes before and after treatment. One-way ANOVA will be used to compare the different extract concentrations across the experimental groups. A P-value of ≤ 0.05 was deemed statistically significant, and all data were presented as mean ± SD.
4. Results
4.1. In vitro Effects on the Parasite
The extract at concentrations of 0.1 and 0.2 mg/mL had no significant effect on L. major promastigotes compared to the control group. However, at concentrations of 0.3 - 0.9 mg/mL, the extract significantly reduced promastigote viability, with complete eradication observed at the highest concentrations (Table 1).
| Pomegranate Peel Extract Concentrations (mg/mL) | Control | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Growth in culture medium (row 1) | ++ | ++ | + | - | - | - | - | - | - | - |
| Growth in culture medium (row 2) | ++ | + | + | - | - | - | - | - | - | - |
| Growth in culture medium (row 3) | ++ | + | - | - | - | - | - | - | - | - |
a ++ indicates a minimal or no observed effect (little or no inhibition).
b + indicates a moderate effect (partial inhibition or moderate impact).
c - indicates a strong inhibitory effect or a significant presence of the observed effect (near total inhibition or major impact).
4.2. In vivo Anti-leishmanial Effects
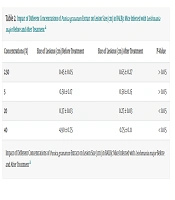
Lesion size measurements before and after treatment revealed that lower extract concentrations (2.5% and 5%) were ineffective in reducing lesion size, as no statistically significant difference was observed when compared to the control group (P > 0.05). Conversely, treatment with 20% and 40% extract concentrations significantly reduced lesion sizes, demonstrating a concentration-dependent therapeutic effect. Mice treated with 20% P. granatum extract exhibited a 27% reduction in lesion size, with the average diameter decreasing from 0.37 cm to 0.27 cm (P < 0.05). The greatest effect was observed in the group treated with 40% extract, in which the lesions decreased by approximately 84%, from 4.90 cm to 0.75 cm (P < 0.05). These results indicate that higher concentrations of P. granatum extract have a significant therapeutic effect on CL and wound healing (Table 2).
| Concentrations (%) | Size of Lesions (cm) Before Treatment | Size of Lesions (cm) After Treatment | P-Value |
|---|---|---|---|
| 2.50 | 0.45 ± 0.05 | 0.65 ± 0.27 | > 0.05 |
| 5 | 0.58 ± 0.17 | 0.58 ± 0.15 | > 0.05 |
| 20 | 0.37 ± 0.03 | 0.27 ± 0.03 | < 0.05 |
| 40 | 4.90 ± 0.75 | 0.75 ± 0.11 | < 0.05 |
a Values are expressed as mean ± SD.
5. Discussion
Pomegranate peel contains various bioactive compounds, including tannins, flavonoids, and organic acids, demonstrating significant pharmacological properties (21). Our study showed that the extract of P. granatum at concentrations of 0.1 and 0.2 mg/mL after 72 h did not inhibit the growth of promastigote forms of L. major. However, this extract at concentrations of 0.3 - 0.9 mg/mL after 72 h significantly decreased the growth rate of promastigote forms and subsequently the viability of promastigote forms compared with the control group. Finally, no live parasites were observed. The ability of P. granatum extract to alter the morphology of promastigotes, even at lower concentrations, underscores its potential mechanism of action, which may warrant further investigation into the specific phytochemicals responsible for this effect. However, previous studies using different extraction methods have reported varying levels of efficacy, which may be attributed to differences in the solubility and bioavailability of active compounds. For example, one study found that hydroalcoholic extracts of pomegranate peel effectively inhibit L. major promastigotes in vitro, likely due to the enhanced solubility of bioactive compounds like tannins, flavonoids, and alkaloids. This method extracts both hydrophilic and lipophilic compounds, resulting in greater bioactivity (19). In contrast, our study focused on the aqueous extract, which, while still demonstrating significant antiparasitic and immunomodulatory effects, shows less pronounced efficacy at lower concentrations compared to the hydroalcoholic extract.
Another study demonstrated that oral administration of P. granatum juice significantly reduced CL lesion size in infected mice, with the therapeutic effects being linked to an increase in antioxidant enzyme activity (20). This suggests that systemic administration of pomegranate-derived compounds may contribute to enhanced immune responses against L. major. In contrast, our study utilized topical application of the aqueous extract, which also led to significant lesion reduction, particularly at 20% and 40% concentrations. The differences in efficacy between oral and topical administration highlight the need for further research to determine the most effective delivery method for P. granatum-based treatments.
Also, these results were consistent with those of previous studies, indicating the anti-leishmanial properties of widely consumed plants and indicating their potential for use as anti-infective drugs. Yousefi et al. (22) showed that the percentage of macrophage viability after 60 h of adding 200 µg/mL of extracts of P. harmala and Alkanna tinctoria was observed to be 80%. Also, Ezatpour et al. (23) showed the anti-promastigote properties of Pistacia khinjuk extract against L. major with a 58.6 ± 3.2 µg/mL.
In the in vivo experiment, it was observed that P. granatum extract at concentrations of 20% and 40% significantly inhibited CL in male BALB/c mice infected with L. major, resulting in recovery rates of 27% and 84%, respectively. Consistent with our findings, research has shown that gum from Pistacia atlantica effectively managed CL in mice infected with L. major (24). Rahimi-Moghaddam et al. (25) reported a notable reduction in both lesion size and parasite load in treated animals compared to those given a placebo or in control groups.
Topical bioavailability is crucial for treating cutaneous and mucocutaneous leishmaniasis with natural products like pomegranate peel extracts. Lipophilic extracts, which penetrate the skin’s lipid-rich outer layer, are more effective for deeper dermal treatment, while aqueous extracts may struggle with skin penetration. However, when enhanced with penetration agents or nanocarriers, aqueous extracts can still be effective (26). Additionally, combining pomegranate peel extracts with conventional drugs may provide a dual approach, leveraging its antioxidant, anti-inflammatory, and immune-stimulating properties to complement traditional treatments and promote wound healing.
The anti-leishmanial effects of P. granatum peel extract in this study can be attributed to its ability to induce oxidative stress in L. major promastigotes. Pomegranate peel extract induces oxidative stress in L. major promastigotes. Bioactive compounds like tannins, flavonoids, and organic acids in the peel extract increase reactive oxygen species (ROS) production, damaging cellular structures and triggering parasite cell death. Additionally, the extract may boost immune responses, including macrophage activation and NO production, further aiding in parasite elimination (27-29). A comparison to Artemisia annua shows similar mechanisms, where ROS generation leads to oxidative damage and cell death in both cases (30).
One of the primary limitations of this study is the lack of a standard anti-leishmanial drug (e.g., glucantime or amphotericin B) as a positive control. Future studies should include a standard anti-leishmanial drug as a positive control to enable direct comparison with established treatments. The study did not quantify the levels of key bioactive compounds, such as punicalagin and ellagic acid, which may be responsible for the observed therapeutic effects. Given that a crude extract was utilized in this study, further investigations are suggested to isolate active chemical constituents that could lead to the development of new treatments for CL. While this study reports the treatment dosages as percentages (e.g., 20%, 40%), converting these to a more universally accepted measure of dosage, such as mg/kg/day, would have facilitated better reproducibility and comparison with other studies. Additionally, the findings are limited to a small animal model, which may not fully replicate clinical conditions. Therefore, further research is needed to assess efficacy in larger, more diverse populations.
5.1. Conclusions
The study shows that the aqueous extract of P. granatum peel exhibits strong anti-leishmanial effects against L. major both in vitro and in vivo, effectively controlling CL in mice. However, further research is needed to compare different extract types (aqueous, hydroalcoholic, and methanolic) in terms of their phytochemical composition, pharmacokinetics, bioavailability, and to isolate the active compounds, optimize treatments, and assess safety and efficacy in clinical settings.