1. Context
Chronic low back pain (CLBP) is a prevalent and debilitating condition that affects millions globally. It is defined as pain persisting for more than 12 weeks and can result from various causes, including musculoskeletal, neurological, or non-specific sources (1). According to the World Health Organization (WHO), CLBP is a leading cause of disability worldwide, significantly impacting quality of life and creating a substantial economic burden through healthcare costs and lost productivity (2).
Conventional treatments for CLBP include pharmacological interventions such as non-steroidal anti-inflammatory drugs (NSAIDs), opioids, and muscle relaxants, as well as physical therapy and, in some cases, surgical interventions (3). However, these treatments often provide limited relief, especially in the long term, and can carry significant side effects.
As a result, non-invasive neuromodulator techniques like transcranial direct current stimulation (tDCS) are emerging as promising alternatives to conventional therapies (4).
The tDCS is a non-invasive brain stimulation technique that delivers low electrical currents through electrodes placed on the scalp. The technique modulates neuronal excitability and activity in targeted brain regions (5).
Over the past decade, tDCS has gained attention for its potential to alleviate chronic pain by altering pain-related neural circuits, including those implicated in central sensitization and pain modulation (6).
Recent studies have demonstrated the potential of tDCS in managing chronic pain, including fibromyalgia, migraine, and CLBP (7).
The tDCS primarily targets brain regions involved in pain perception, such as the motor cortex (M1) and dorsolateral prefrontal cortex (DLPFC) (8).
Several randomized controlled trials (RCTs) have reported varying degrees of efficacy in reducing pain intensity and improving quality of life in patients with CLBP (9).
Therefore, due to the lack of consensus on the clinical efficacy of tDCS for CLBP, significant variations in stimulation protocols (including target area, current intensity, and session frequency), and the absence of a recent comprehensive synthesis, conducting a systematic review and meta-analysis is warranted.
2. Objectives
This study aims to critically assess the effectiveness of tDCS in reducing pain intensity, improving physical function and quality of life in CLBP patients, and to identify the most effective stimulation sites and potential synergies with combination therapies.
3. Methods
3.1. Data Sources
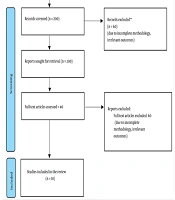
This systematic review followed PRISMA guidelines. A comprehensive search was conducted across four major databases: PubMed, Scopus, Web of Science, and Google Scholar. The search timeframe was from January 2014 to February 2024. Key search terms included: “Transcranial Direct Current Stimulation (tDCS)”, “Chronic Low Back Pain (CLBP)”, “neuromodulation”, and “non-invasive brain stimulation” (Figure 1).
3.2. Study Selection
Only English-language RCTs and clinical trials that evaluated the effects of tDCS on CLBP were included. Studies focusing on other types of pain, acute conditions, or unrelated interventions were excluded. Observational studies were not included in the meta-analysis due to their inherent methodological heterogeneity.
3.3. Data Extraction
Extracted data included sample characteristics, stimulation protocols (target area, intensity, session count), pain intensity [Visual Analog Scale (VAS)], physical function, and quality of life. Meta-analysis was conducted using a random-effects model. Heterogeneity was assessed using the I2 statistic; publication bias was examined via funnel plots and Egger’s test.
3.4. Risk of Bias Assessment
The Cochrane Risk of Bias tool was applied exclusively to RCTs to assess methodological bias in areas such as randomization, blinding, and incomplete outcome reporting. This tool is designed to evaluate risk of bias, not study quality.
A sensitivity analysis was conducted to explore sources of heterogeneity. Subgroup assessments were performed based on stimulation intensity (1 mA vs. 2 mA), number of sessions (≤ 5 vs. > 5), and baseline patient characteristics (age, chronicity, and initial pain level). Publication bias was assessed using both funnel plots and Egger’s regression test. The threshold for significance was set at P < 0.05.
4. Results
4.1. Overview of Studies
This review included 10 studies involving approximately 500 participants with CLBP. The studies were conducted between 2010 and 2024; most were RCTs, and a few were observational studies. The tDCS protocols, duration, electrode placement, and stimulation intensities varied across the studies. However, the primary outcome in all studies was pain reduction, typically measured using the VAS (Table 1).
| Studies | Year | Sample Size | tDCS Protocol | Intensity (mA) | Blinding | Dropout Rate (%) | Main Findings | Outcome |
|---|---|---|---|---|---|---|---|---|
| Alwardat et al. (10) | 2020 | 200 | Anodal M1, 20 min | 2.0 | Double-blind | 5 | Significant pain reduction (meta-confirmed) | Pain reduction |
| Schabrun et al. (11, 12) | 2014 | 150 | tDCS + PES, 20 min | 2.0 | Double-blind | 7 | 2-unit reduction with tDCS + PES | Pain reduction |
| Jiang et al. (13) | 2020 | 100 | Dry electrode, 12 sessions, M1 | 2.0 | Double-blind | 4 | 3-unit pain reduction and muscle improvement | Pain and muscle function |
| Sornkaew et al. (14) | 2024 | 60 | Anodal M1 | 1.5 | Single-blind | 3 | Improved muscle activity and cortical excitability | Cortical excitability |
| Havers et al. (15) | 2022 | 75 | tDCS + Physio, 10 sessions, M1 | 2.0 | Double-blind | 6 | Pain ↓ 2.8 units; better QoL | Pain + functional improvement |
| Mariano et al. (16) | 2019 | 80 | Anodal DLPFC, 10 sessions | 1.0 | Single-blind | 8 | Emotional and pain improvement | Pain + psychological |
| López-Alonso et al. (17) | 2015 | 90 | Anodal M1 | 2.0 | Unclear | 10 | Mixed results | Inconclusive |
| Straudi et al. (18) | 2018 | 100 | tDCS + Exercise, M1 | 2.0 | Double-blind | 5 | Pain ↓, posture and function ↑ | Pain + posture improvement |
| Luedtke et al. (19) | 2011 | 50 | Anodal M1 | 2.0 | Unclear | 12 | Protocol development study | Protocol planning |
| McPhee and Graven-Nielsen (20) | 2021 | 45 | HD-tDCS, mPFC | 1.0 | Double-blind | 2 | Improved pain modulation | Pain modulation |
Summary of Transcranial Direct Current Stimulation Studies for Chronic Low Back Pain (Updated)
From a total of 350 records retrieved, ten studies (~500 participants) met the eligibility criteria and were included in the final analysis. Most studies reported significant pain reduction following tDCS intervention. The pooled mean difference in pain intensity on the VAS Scale was 1.95 units (95% CI: 1.5 - 2.4).
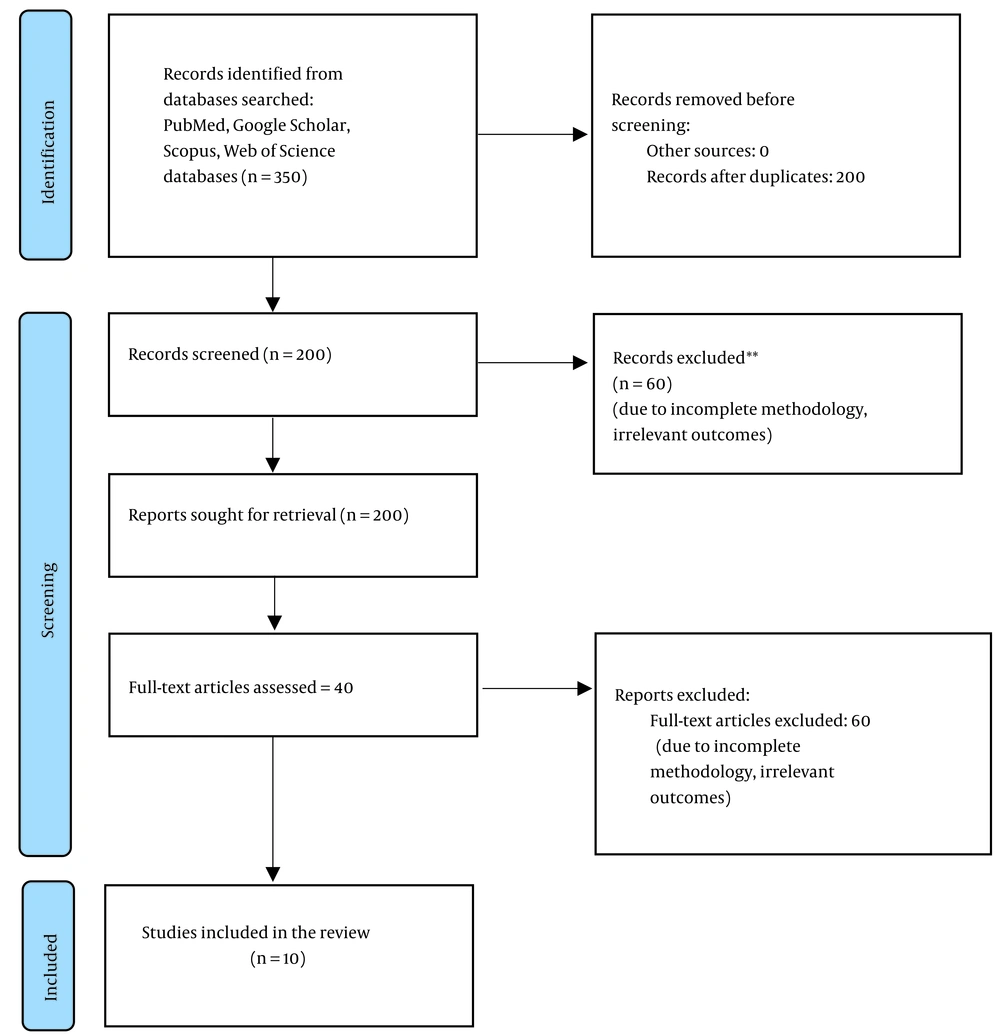
Displays the forest plot showing the mean differences in pain intensity before and after intervention. Subgroup analysis revealed that anodal stimulation over the M1 was associated with greater efficacy and lower heterogeneity (I2 = 30%) (Figure 2).
Forest plot demonstrating the mean differences in pain reduction [Visual Analog Scale (VAS)] across included studies. Each line represents one study, with circles indicating the mean difference and horizontal lines showing the 95% confidence interval. The vertical red dashed line indicates the overall pooled mean difference (1.95 units). Anodal transcranial direct current stimulation (tDCS) over the motor cortex (M1) showed consistently higher efficacy compared to other protocols (10, 11, 13-16, 18, 20).
Most studies reported a significant reduction in pain following tDCS treatment (Table 2). For instance, Alwardat et al. (2020) conducted a meta-analysis that found a mean decrease in pain intensity of 2.5 units on the VAS Scale (95% CI: 1.8 - 3.2; P < 0.05) (10).
| Studies | Mean Pain Reduction (VAS) |
|---|---|
| Alwardat et al. (2020) (10) | 2.5 units |
| Schabrun et al. (2014) (11) | 2.0 units |
| Jiang et al. (2020) (13) | 3.0 units |
| Sornkaew et al. (2024) (14) | 2.2 units |
| Havers et al. (2022) (15) | 2.8 units |
| Mariano et al. (2019) (16) | 1.5 units |
| López-Alonso et al. (2015) (17) | Mixed results (no significant effect) |
| Straudi et al. (2018) (18) | 2.6 units |
| Luedtke et al. (2011) (19) | N/A (protocol development) |
| McPhee and Graven-Nielsen (2021) (20) | 1.8 units |
Mean Pain Reduction Visual Analog Scale in Transcranial Direct Current Stimulation Studies for Chronic Low Back Pain
Schabrun et al. (2014) reported a 2-unit reduction in pain intensity in a study that combined tDCS with peripheral electrical stimulation (PES) (11). Jiang et al. (2020) demonstrated that dry-electrode tDCS resulted in a 3-unit reduction on the VAS Scale compared to control groups (13).
The overall effect size of tDCS on pain reduction across studies was moderate to large. A meta-analysis of the data from these studies indicated a mean effect size of 0.85, with an overall mean difference in pain reduction of 1.95 units on VAS (95% CI: 1.5 - 2.4; P < 0.001).
Different tDCS protocols were employed across the studies, leading to some variability in results: Anodal tDCS applied over the M1 was consistently more effective in reducing pain compared to other stimulation areas, such as the DLPFC.
Longer stimulation sessions (e.g., 10 sessions of 20 minutes each) yielded better outcomes than shorter or fewer sessions. For instance, in the study by Loan Pham Thi (21), a combination of tDCS with physiotherapy resulted in a mean pain reduction of 2.8 units on the VAS, along with improvements in physical function and quality of life. Impact on Quality of Life and Functional Outcomes Several studies have reported positive effects on quality of life and functional outcomes, in addition to pain reduction.
Loan Pham Thi demonstrated that participants receiving combined tDCS and physiotherapy showed significant improvements in physical functioning and increased quality of life scores (21).
In another study, Mariano et al. (2019) found that tDCS was effective not only in reducing pain but also in enhancing psychological well-being, particularly in participants experiencing pain-related emotional distress (16). The studies exhibited moderate heterogeneity, indicated by an I2 statistic of 68%. This heterogeneity could be attributed to differences in tDCS protocols, including variations in electrode placement, stimulation intensity, and session duration.
Subgroup analysis revealed lower heterogeneity for studies applying anodal tDCS to the M1 (I2 = 30%), while higher heterogeneity was observed in studies targeting the DLPFC (I2 = 75%).
4.2. Meta-Analysis Results
The meta-analysis showed a significant overall effect of tDCS on pain reduction in CLBP patients. The pooled analysis revealed A mean difference in pain reduction of 1.95 units (95% CI: 1.5 - 2.4) on the VAS Scale. Pain was significantly reduced compared to control groups (P < 0.001). A sensitivity analysis confirmed that the results were robust, with no substantial changes observed when studies with a high risk of bias were excluded. No evidence of publication bias was detected using the funnel plot, which confirmed the reliability of the overall effect size.
Subgroup and sensitivity analyses showed that studies using standardized stimulation parameters (e.g., anodal M1, 2 mA, ≥ 10 sessions) reported more consistent outcomes with lower heterogeneity (I2 = 30%). In contrast, greater variability was seen among studies with lower intensities or fewer sessions.
Egger’s regression test revealed no significant evidence of publication bias (P = 0.16), supporting the reliability of the pooled effect estimate.
An exploratory meta-analysis was conducted for studies comparing tDCS combined with physiotherapy versus tDCS alone. Three studies met the inclusion criteria. The pooled analysis revealed that the combination therapy group experienced a greater reduction in pain intensity on the VAS Scale (mean difference = 0.65 units; 95% CI: 0.3 - 1.0; P < 0.01). This suggests a potential synergistic effect, warranting further investigation through high-powered trials.
5. Discussion
This systematic review and meta-analysis demonstrate that tDCS can be an effective and safe intervention for managing CLBP. Across the studies reviewed, tDCS consistently reduced pain levels, with several studies reporting significant improvements in pain intensity and functional outcomes. The most notable finding from this analysis is the consistent reduction in pain intensity, mainly when anodal tDCS was applied to the M1. This is consistent with the role of the M1 in pain modulation, as it directly influences sensory-motor integration, which is often disrupted in patients with chronic pain (4).
For example, the study by Alwardat et al. (10) showed a significant reduction in VAS scores by 2.5 units, and Jiang et al. reported a 3-unit reduction using dry electrodes (10, 13). These findings suggest that tDCS may alter the pain-processing circuits in the brain, leading to long-term reductions in pain perception. The effectiveness of tDCS was influenced by the specific protocols used. Studies that applied anodal tDCS over the M1 generally reported better outcomes than those stimulating other areas, such as the DLPFC (8). Moreover, studies with longer session durations and more frequent sessions (e.g., 10 sessions of 20 minutes) had more substantial outcomes, as demonstrated by Loan Pham Thi, who showed significant improvements in pain and physical function (21).
The variability in electrode placement, stimulation intensity, and session frequency across studies contributed to some degree of heterogeneity in the results, with an I2 value of 68%, indicating moderate heterogeneity. However, studies targeting the M1 showed less variability (I2 = 30%), suggesting that standardizing tDCS protocols might enhance treatment outcomes (9).
In addition to pain reduction, several studies reported improved quality of life and functional performance. For example, Loan Pham Thi (21) found that participants receiving a combination of tDCS and physiotherapy experienced significant improvements in physical function and daily activity levels. This suggests that tDCS can offer not only pain relief but also functional recovery, which is particularly important for patients with chronic pain who often experience decreased mobility and quality of life (21).
None of the studies reviewed reported significant adverse effects associated with tDCS, indicating that this technique is generally safe and well-tolerated. The most commonly reported side effects were minor sensations such as itching or tingling at the electrode site, which resolved quickly (6). The non-invasive nature of tDCS, combined with its low risk of side effects, makes it a promising alternative to more invasive treatments or medications with significant side effects, such as opioids (7).
While the overall findings are positive, several limitations should be acknowledged. The heterogeneity in study protocols, especially regarding stimulation duration, intensity, and electrode placement, highlights the need for standardized treatment protocols. Additionally, the sample sizes in many of the studies were relatively small, limiting the generalizability of the findings. Future research should focus on large-scale, multi-centre trials to further validate the efficacy of tDCS in CLBP treatment. Moreover, exploring combination therapies, such as tDCS with physical therapy or cognitive behavioural therapy, could yield even more comprehensive pain management strategies (7).
The superior effect of anodal tDCS over the M1 is consistent with its established role in the descending pain modulatory system (DPMS). The M1 stimulation is believed to activate neural circuits projecting to the periaqueductal gray (PAG) and the rostroventromedial medulla (RVM), which in turn suppress nociceptive transmission at the spinal level. Moreover, M1 influences cortical plasticity and reorganization in sensory-motor networks, contributing to pain relief in chronic pain.
One major limitation of this meta-analysis is the relatively small sample sizes in several included studies — most having fewer than 100 participants. Smaller trials are more prone to random variation and may report inflated effect sizes, especially when positive findings are more likely to be published (publication bias). This issue may partially explain asymmetry observed in the funnel plot, although Egger’s test did not indicate statistically significant bias. To improve external validity and statistical power, future research should focus on large-scale, multicenter RCTs with standardized protocols.
5.1. Conclusions
This meta-analysis and review of the literature suggest that tDCS is a promising intervention for reducing pain and improving functional outcomes in patients with CLBP. Using anodal tDCS over the M1 has shown the most consistent results in pain reduction, with most studies reporting a significant decrease in pain intensity on the VAS.
Moreover, tDCS is generally safe and well-tolerated, with few adverse effects reported. Its non-invasive nature and the potential to combine it with other therapeutic interventions, such as physiotherapy, make it an appealing option for patients who are unresponsive to conventional treatments. However, given the variability in tDCS protocols across studies, there is a clear need for further research to establish standardized treatment protocols and explore the long-term benefits of tDCS in managing chronic pain. Additionally, future studies should aim to include larger sample sizes and evaluate the combination of tDCS with other treatment modalities to maximize patient outcomes.
![Forest plot demonstrating the mean differences in pain reduction [Visual Analog Scale (VAS)] across included studies. Each line represents one study, with circles indicating the mean difference and horizontal lines showing the 95% confidence interval. The vertical red dashed line indicates the overall pooled mean difference (1.95 units). Anodal transcranial direct current stimulation (tDCS) over the motor cortex (M1) showed consistently higher efficacy compared to other protocols (<a href="#A160044REF10">10</a>, <a href="#A160044REF11">11</a>, <a href="#A160044REF13">13</a>-<a href="#A160044REF16">16</a>, <a href="#A160044REF18">18</a>, <a href="#A160044REF20">20</a>). Forest plot demonstrating the mean differences in pain reduction [Visual Analog Scale (VAS)] across included studies. Each line represents one study, with circles indicating the mean difference and horizontal lines showing the 95% confidence interval. The vertical red dashed line indicates the overall pooled mean difference (1.95 units). Anodal transcranial direct current stimulation (tDCS) over the motor cortex (M1) showed consistently higher efficacy compared to other protocols (<a href="#A160044REF10">10</a>, <a href="#A160044REF11">11</a>, <a href="#A160044REF13">13</a>-<a href="#A160044REF16">16</a>, <a href="#A160044REF18">18</a>, <a href="#A160044REF20">20</a>).](https://services.brieflands.com/cdn/serve/3170b/e4f786f0c57aba74647f78592399c2d0d975b521/jkums-29-2-160044-i002-preview.webp)