1. Background
Infection with hepatitis C virus (HCV) is a public health issue worldwide. The infection is associated with deleterious complications such as liver cirrhosis and hepatocellular cancer (1). It is estimated that approximately 150 million individuals globally are living with a chronic HCV infection, leading to over 500,000 deaths each year (1). The development of direct-acting antivirals (DAAs) has revolutionized HCV treatment, making it possible to realistically achieve viral eradication (2). Effective therapy resulting in a sustained virologic response (SVR) greatly lessens the likelihood of severe complications such as cirrhosis and liver cancer (2, 3). The potential for a late relapse after therapy concludes, however, remains one of the persistent challenges in treating HCV (4).
Multiple studies in Iraq showed that infection with HCV remains consistently low (5). The prevalent genotype of HCV in the nation is genotype 1 (G1), with genotype 4 (G4) next in frequency (5). Additionally, research carried out in Iraq has indicated remarkably high SVR rates in individuals with hemoglobinopathies and advanced renal disease (3, 6). However, there is a distinct lack of longitudinal data assessing relapse beyond the typical 12 - 24 month follow-up window. Considering the significance of long-term treatment outcomes, this research intends to evaluate the rate of HCV relapse 84 months following the end of therapy, offering valuable insight into the durability of SVR in the Iraqi population. Given the rising availability of DAAs and the global push toward HCV elimination, understanding long-term treatment efficacy in local settings is essential.
2. Objectives
The study’s findings could help refine national HCV treatment guidelines, optimize post-treatment monitoring protocols, and ultimately support public health planning in Iraq.
3. Methods
3.1. Patients
Twelve weeks after the completion of DAA therapy, a SVR was confirmed by a negative HCV RNA result using real-time polymerase chain reaction (RT-PCR). The follow-up period extended for 84 months. Patients with a prior history of HCV infection who had achieved SVR were invited for annual follow-up through HCV RT-PCR testing. Active follow-up was conducted through scheduled telephone calls and face-to-face visits to ensure continued monitoring and patient engagement. The mean age of the participants was 23 ± 7 years.
3.2. Inclusion and Exclusion Criteria
Patients diagnosed with HCV who received DAA therapy, achieved a sustained virological response, agreed to participate, and were available for 84-month follow-up with annual HCV RT-PCR tests were included. Patients treated with interferon-based regimens and those with irregular follow-up were excluded from the study.
3.3. Serological Testing for Hepatitis B Surface Antigen and HIV
At the beginning of the treatment and annually thereafter, patients were screened for HIV and hepatitis B surface antigen (HBsAg) positivity. A commercial ELISA kit (DIA.PRO Diagnostic Bioprobes, Italy) was used to examine patient samples to determine the presence of HIV and the HBsAg.
3.4. Hepatitis C Virus RNA Extraction
The QIAamp RNA Extraction Kit (Qiagen) was used to extract HCV RNA from patient samples in accordance with the manufacturer's instructions for automated extraction using the QIAcube system (Qiagen). A NanoDrop spectrophotometer was then used to measure the concentration and purity of the RNA.
3.5. Hepatitis C Virus RNA Quantification and Genotyping
Using a Rotor-Gene Q thermocycler and the Artus HCV RG-RT PCR Kit, HCV RNA amplification was carried out. The detection range of the test was 65 to 106 IU/mL. Positive samples were further examined for genotyping using the GEN-C 2.0 reverse hybridization strip assay (Nuclear Laser Medicine, Settala, Italy). This technique distinguishes between various HCV genotypes by identifying sequence changes in the 5'-UTR and core sections of the viral genome.
3.6. Ethical Consideration
The study was approved by the Ethics Committee of the College of Medicine, University of Zakho (approval no. 17/UOZE37). Informed consent was obtained from all participants prior to inclusion in the study. Participants were clearly informed about the purpose of the research, the procedures involved, and their right to withdraw at any time without any consequences to their medical care. All patient data were anonymized and stored securely to prevent unauthorized access. Personal identifiers were removed from the dataset, and results were presented in aggregate to ensure that no individual could be identified.
4. Results
4.1. Patients
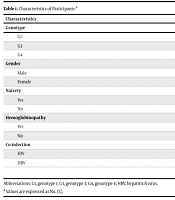
A total of 89 patients were included in the study, all of whom received DAA treatment. Patients with G1 and 4 received a combination of sofosbuvir and ledipasvir, while patients with genotype 3 (G3) received sofosbuvir and daclatasvir. The study population's gender distribution revealed that 37 (41.57%) were female and 52 (58.43%) were male. The demographic and clinical characteristics of these patients are described in Table 1.
| Characteristics | Values |
|---|---|
| Genotype | |
| G1 | 41 (46.07) |
| G3 | 7 (7.87) |
| G4 | 41 (46.07) |
| Gender | |
| Male | 52 (58.43) |
| Female | 37 (41.57) |
| Naivety | |
| Yes | 87 (97.78) |
| No | 2 (2.22) |
| Hemoglobinopathy | |
| Yes | 10 (11.24) |
| No | 79 (88.76) |
| Co-infection | |
| HIV | 0 (0) |
| HBV | 0 (0) |
Abbreviations: G1, genotype 1; G3, genotype 3; G4, genotype 4; HBV, hepatitis B virus.
a Values are expressed as No. (%).
4.2. Genotype Distribution
The distribution of HCV genotypes among the 89 patients was as follows: Forty-one (46.07%) had G1 infection, 7 (7.87%) had G3, and 41 (46.07%) had G4 (Table 1).
4.3. Treatment Naivety
Regarding past treatment history, 87 (97.75%) had never received antiviral therapy before, making them treatment-naive. Just two individuals (2.25%) had received antiviral therapy before.
4.4. Co-morbidities and Co-infection
Additionally, the patients' co-morbidities were evaluated. While 79 (88.76%) had no substantial co-morbidities, 10 (11.24%) had thalassemia. Although most patients in the group had no other significant medical disorders, thalassemia was the most prevalent co-morbidity found. In terms of other infections, none of the study participants had co-infections with either HIV or the hepatitis B virus (HBV). There were no co-infections of HIV or HBV in the cohort; none of the patients tested positive for either infection.
5. Discussion
The HCV infection is a major public health issue, especially in developing nations with limited resources such as Iraq. The infection can lead to deleterious complications, such as liver cirrhosis and hepatocellular carcinoma (1, 2). Timely identification and efficient intervention are vital in averting these outcomes (1, 2). While earlier studies have indicated a low prevalence of HCV in Iraq, the arrival of highly effective DAA treatments has been a significant advancement toward eliminating the virus in the nation (5).
The possibility of recurrence, which may appear as reinfection following treatment or as a delayed relapse after reaching SVR, is one challenge to expanding HCV treatment. Individuals who participate in ongoing high-risk activities, such as injecting drug users (IDUs), are particularly vulnerable due to their increased likelihood of reinfection. A meta-analysis study showed that the 5-year recurrence risk for HCV low-risk patients was 0.95%, with a pooled recurrence rate of 1.85 per 1000 person-years of follow-up (PYFU). On the other hand, the 5-year recurrence risk for high-risk patients was 10.67% due to a considerably higher pooled recurrence rate of 22.32 per 1000 PYFU (7). In our study, none of the patients who finished DAA treatment had a recurrence during the follow-up period. Our findings contrast with several previous studies reporting recurrence rates in HCV patients. For example, a study by Huang et al. found that for low-risk patients, the pooled recurrence rate was 0.89/1000 PYFU. For high-risk patients, the pooled recurrence rate was 29.37/1000 PYFU (8). The differences between our study and other studies can be explained by human genetic makeup, viral genotypes, and the treatment regimen used.
Additionally, our research supports other studies that emphasized that the lack of coinfections can significantly predict long-term treatment success (9, 10). Furthermore, efficient post-treatment management and compliance with preventive measures may have played a role in the zero-reinfection rate observed in our group.
Our study has limitations. Firstly, we recruited patients without high-risk behaviors, which may limit generalizability. However, the study allowed for a focused analysis of relapse in a well-defined group. Secondly, the small sample size and lack of patient diversity make it difficult to generalize the results. However, we included all the patients we received during the study period, and we believe that it reflects real-world outcomes with minimal selection bias. Finally, being a single-center study may limit external applicability, but the study provides important long-term data from a unique population.
5.1. Conclusions
Our research showed that there were no instances of HCV recurrence or reinfection in patients who received treatment with DAAs. These results underscore the strong effectiveness of DAAs in attaining a SVR and avoiding recurrence. The findings are optimistic, despite the limited sample size and the possibility that our group represents a lower-risk subset of HCV patients. Future research with larger and more diverse populations is warranted to confirm these results and to further elucidate the factors that contribute to the durability of SVR over extended periods. Further investigation involving larger groups and extended follow-up times is crucial to better understand the long-lasting effects of DAAs in the Iraqi population. In particular, studies that include patients with co-morbidities and HBV co-infection would be valuable. Additionally, genomic and immunological studies may help identify host or viral factors associated with long-term treatment success or relapse. Future research with larger sample sizes, particularly targeting high-risk populations, could help to enhance these findings.