1. Background
Aluminum (Al) is the third element in the earth’s crust and the most abundant metal (after iron and calcium) widely distributed in the environment (1). This metal can be found in almost all food crops such as corn, yellow cheese, tea, salts, herbs, and spices. In addition, it is found in the mixture of a group of drugs and applied in drinking-water treatment (2). Thus, the entire population is largely exposed to it (1).
Al causes a wide range of toxicity in the nervous, hematopoietic, skeletal, respiratory, and immune systems (3, 4).
By non-controlled production of free oxygen radicals, Al leads to lipid peroxidation resulting in damage to cellular membranes and mitochondrial phospholipids (5). This metal consolidates superoxides by forming AlO2-, and facilitates biological oxidation with the formation of hydrogen peroxide Fe2+. Also, by producing superoxide anions via the Fenton reaction by converting Fe3+ to Fe2+, it changes antioxidant enzyme activities in the cells. So, Al cytotoxicity is known by its role in stimulating oxidative stress (6).
Some chelators such as deferoxamine (DFO) and deferiprone (DFP) have been found to decrease Al levels in tissues and the oxidative stress caused by it; but these compounds create inevitable side effects like agranulocytosis, anorexia, and liver fibrosis (1).
Today, the protective effects of natural products with antioxidant properties, such as ginger, saffron, and honey, have been investigated in aluminum toxicity (7), but few studies have been carried out on the effects of probiotics on aluminum toxicity.
Probiotics are living microorganisms that exert their useful effects on the health of the host if consumed adequately. These microorganisms are a part of the normal flora of the digestive system. Lactobacillus, bifidobacteria, and a group of yeasts are considered the most important probiotics. Destroying pathogens, producing antimicrobial compounds, improving the intestinal microbial balance, enhancing lactose digestion, as well as absorbing minerals such as calcium and iron are the characteristic of probiotics (8-10).
Probiotics are also beneficial for the human body because they produce vitamin-like compounds including B-vitamins (11). Some evidence has demonstrated that probiotics increase the intestine’s iron absorption ability by creating an acidic environment (10).
Protection against oxidative stress and the ability to decrease the risk of accumulation of reactive oxygen metabolites have also been reported among the positive effects of probiotics (12).
For example, the increased activity of a group of antioxidant enzymes, particularly superoxide dismutase, was observed in the liver and kidneys of cadmium-infected rats after consuming a dietary intake of Lactobacillus plantarum and Bacillus coagulans (13). In another similar research, the strengthening of the antioxidant system was seen in mercury-infected rats after taking these two probiotics (14).
The antioxidant mechanism of probiotics could be related to free oxygen radicals scavenging, removal of metal ions, inhibition of oxidant compounds, and prevention of their production (12).
Selenium (Se) is an essential trace element in the human diet showing the same antioxidant properties as probiotics. This metal micronutrient is the major constituent of selenoenzymes such as glutathione peroxidase (GPx3, GPx2), thioredoxin reductase, and selenoproteins P (15), which protect animal cells from oxidative damages (16).
Se is available in both organic and inorganic forms. Studies have shown that the organic form of Se has higher absorption potentials and less toxicity than its inorganic form. Therefore, using bioseleniums such as selenomethionine, probiotics, and Se-enriched plants as dietary supplements have been considered (17). A study by El-Bayoumy and colleagues carried out on a group of adult men showed that consuming Se-enriched yeast not only improved testosterone secretions, but also significantly increased the level of reduced glutathione in their blood as compared to the control group, which indicated a decline of oxidative stress levels in their bodies (18).
As the positive impacts of Se dietary supplements and probiotics in reducing oxidative stress levels by strengthening the antioxidant defense system have been specified (19), the present study investigated the likely impact of the Saccharomyces boulardii and S. boulardii enriched with Se against the AlCl3-induced toxicity in rats.
2. Methods
Thirty-six adult female Wistar rats weighing 120 - 140 g each were obtained from the animal house of the Islamic Azad University of Falavarjan and kept under favorable environmental conditions of 20 - 25°C, humidity 53 ± 2%, and 12 hours light/dark period. After a week, in order to compromise with the environmental conditions, they were allocated into 6 groups as follows:
A. Injection control group: 1 cc intradigestive injection of distilled water for 5 weeks (every other day) and intraperitoneal injection of 0.5 cc distilled water at the end of the third week.
B. S. boulardii treatment group: 1 cc intradigestive injection of yeast suspension (1.5 × 108 CFU/mL) (20) for 5 weeks (every other day) and intraperitoneal injection of 0.5 cc distilled water at the end of the third week.
C. Se-enriched S. boulardii treatment group (sodium selenite): 1 cc intradigestive injection of yeast suspension enriched with Se for 5 weeks (every other day) and intraperitoneal injection of 0.5 cc distilled water at the end of the third week.
D. AlCl3-infected group: 1 cc intradigestive injection of distilled water for 5 weeks and intraperitoneal injection of 0.5 cc of AlCl3 at the end of the third week.
E. S. boulardii treated and AlCl3 infected group: 1 cc intradigestive injection of yeast suspension (1.5 × 108 CFU/mL) for 5 weeks (every other day) and intraperitoneal injection of 0.5 cc of AlCl3 (4 mg/kg) (21) at the end of the third week.
F. Se-enriched S. boulardii treated and AlCl3 infected group: 1 cc intradigestive injection of yeast suspension enriched with Se for 5 weeks (every other day) and intraperitoneal injection of 0.5 cc of AlCl3 at the end of the third week.
In order to enrich the yeast with Se, 90 mL of sodium selenite (10 mg/mL) was added to 100 cc of suspension prepared from yeast and incubated for 48 hours at 37°C. After incubation in the 3000 rpm, it was centrifuged for 15 minutes and washed with sterile normal saline twice to remove excess selenium. Then, the Se-enriched yeast suspension (1.5 × 108 CFU/mL) was prepared from the resulting sediments (22).
Finally, at the end of the treatment period, the rats were weighed and blood samples taken straight from their hearts.
The number and index of blood cells (CBC) were measured using the cell counter device Mindray BC 6800 model.
The amount of serum iron was calculated according to the instructions of Artiss and colleagues (23). The modified method of Goodwin and colleagues (24) and TIBC assay kit made by Pars Azmun Company were used to measure TIBC (total iron binding capacity).
The blood’s total antioxidant capacity (T-AOC) was analyzed with the ELISA Reader method using the T-AOC assay kit of EASTBIOPHARM Company.
Malondialdehyde (MDA) levels were evaluated using the thiobarbituric acid (TBA) method based on the detection of MDA-TBA levels at the wavelength of 532 nm according to the instructions of Qiao and colleagues (25).
2.1. Statistical Analysis
The data was analyzed as mean ± standard deviation using the SPSS-18 statistical software.
3. Results
According to Table 1, a significant increase in the number of white blood cells (WBC) was seen in the group exposed to AlCl3 as compared to the control group (P ≤ 0.05).
| Parameter | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | S. boulardii | Se-Enriched, S. boulardii | AlCl3 | AlCl3-, S. boulardii | AlCl3-, Se-Enriched, S. boulardii | |
| WBC, 103/µL | 7.33 ± 0.803 | 7.49 ± 2.253 | 7.53 ± 0.787 | 9.88 ± 1.370* | 7.08 ± 1.054# | 6.57 ± 1.436# |
| PLT, 103/µL | 7.24 ± 80.011 | 7.36 ± 40.784 | 7.59 ± 118.740 | 7.65 ± 60.384 | 6.46 ± 44.777 | 7.95 ± 64.700 |
| RBC, 106/µL | 8.27 ± 0.450 | 8.23 ± 0.387 | 7.94 ± 0.539 | 7.92 ± 0.482 | 8.29 ± 0.535 | 8.17 ± 0.495 |
| MCV, fL | 57.15 ± 1.573 | 57.15 ± 1.535 | 55.88 ± 1.858 | 50.65 ± 3.083*** | 58.25 ± 2.630# | 54.88 ± 2.451# |
| MCH, pg | 17.53 ± 0.338 | 17.46 ± 0.706 | 17.26 ± 0.747 | 17.33 ± 0.393 | 18.23 ± 0.592 | 16.86 ± 0.659 |
| MCHC, g/dL | 31.3 ± 0.729 | 30.56 ± 0.612 | 30.88 ± 0.435 | 30.9 ± 0.579 | 31.28 ± 0.775 | 30.71 ± 0.435 |
| RDW, % | 13.68 ± 0.426 | 14.43 ± 0.952 | 14.4 ± 0.764 | 16.8 ± 3.343* | 14.28 ± 0.487# | 15.8 ± 1.660# |
| HG, g/dL | 14.46 ± 0.628 | 14.38 ± 0.444 | 13.65 ± 0.417 | 13.9 ± 0.583 | 14.96 ± 0.520 | 13.8 ± 1.138 |
| HCT, % | 46.85 ± 1.793 | 47.06 ± 1.501 | 43.93 ± 1.774 | 45.75 ± 0.564 | 47.38 ± 0.970 | 44.9 ± 3.834 |
WBC numbers decreased significantly (P ≤ 0.05) in both infected groups treated with S. boulardii and Se-enriched S. boulardii as compared to the Al-infected group.
There were no significant differences in the platelet numbers between treatment groups as compared to the control group.
Also, no significant differences were observed in the number of red blood cells, indices MCH and MCHC, hemoglobin concentrations, and hematocrit percentages among the groups; but, in the AlCl3-infected group, MCV (mean corpuscular volume) decreased significantly (P ≤ 0.001) and RDW (P ≤ 0.05) increased.
These two parameters in both infected groups treated with S. boulardii and Se-enriched S. boulardii increased respectively (P ≤ 0.05) and decreased (P ≤ 0.05) as compared to the Al-infected group.
As shown in Figure 1, iron concentrations in the AlCl3-infected group had a significant reduction (P ≤ 0.05), while it increased significantly in the S. boulardii and enriched S. boulardii treatment groups as compared to the control group (P ≤ 0.001).
Comparison of serum iron concentrations between experimental groups. The results are expressed as mean ± SD. The asterisks indicate the significance levels of experimental groups as compared to the control group and # represents a comparison of the two last groups with the Al-infected group. Levels of significance values are *P ≤ 0.05, ***P ≤ 0.001, #P ≤ 0.05 ##P ≤ 0.01.
Iron concentrations significantly increased in both infected groups treated with S. boulardii and Se-enriched S. boulardii (P ≤ 0.01 and P ≤ 0.05, respectively) as compared to the Al-infected group.
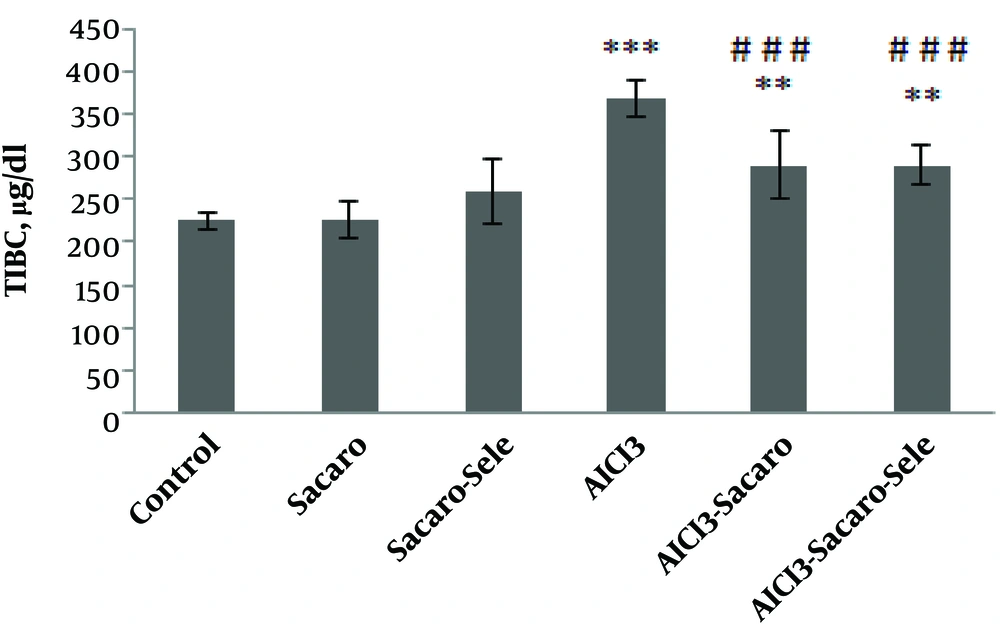
As shown in Figure 2, TIBC in the AlCl3-infected group, S. boulardii treated and AlCl3 infected group, and Se-enriched S. boulardii treated and AlCl3 infected group showed significant increases (P ≤ 0.001 and P ≤ 0.01, respectively).
Comparison of serum TIBC concentrations between experimental groups. The results are expressed as mean ± SD. The asterisks indicate the significance levels of experimental groups as compared to the control group and # represents a comparison of the last two groups with the Al-infected group. Levels of significance values are **P ≤ 0.01, ***P ≤ 0.001, ###P ≤ 0.001.
TIBC significantly declined (P ≤ 0.001) in both infected groups treated with S. boulardii and Se-enriched S. boulardii as compared to the Al-infected group.
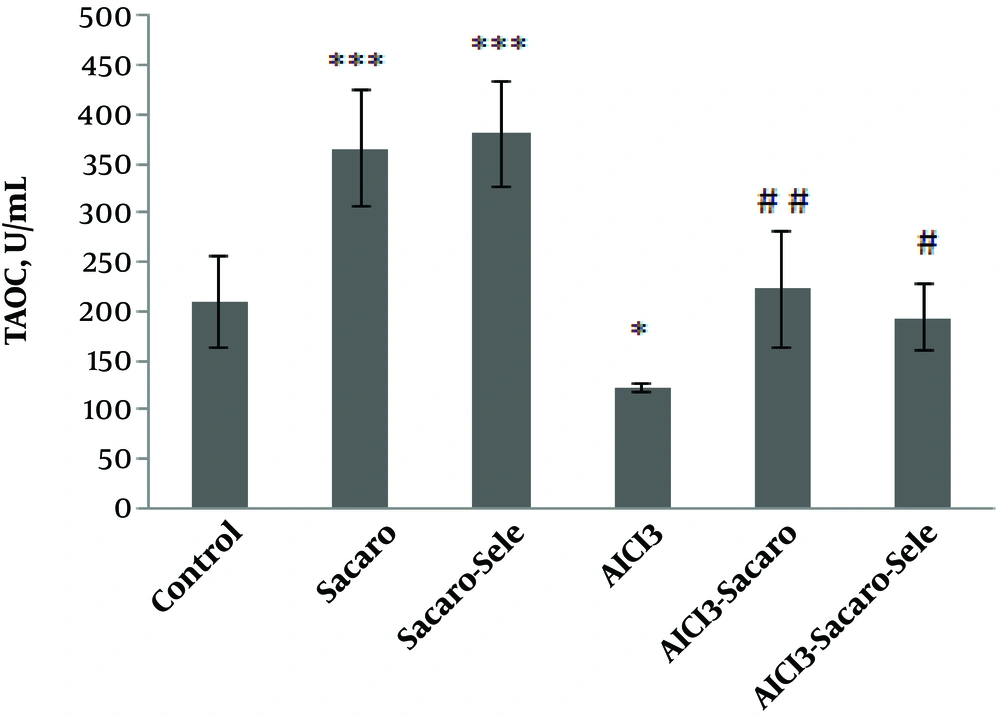
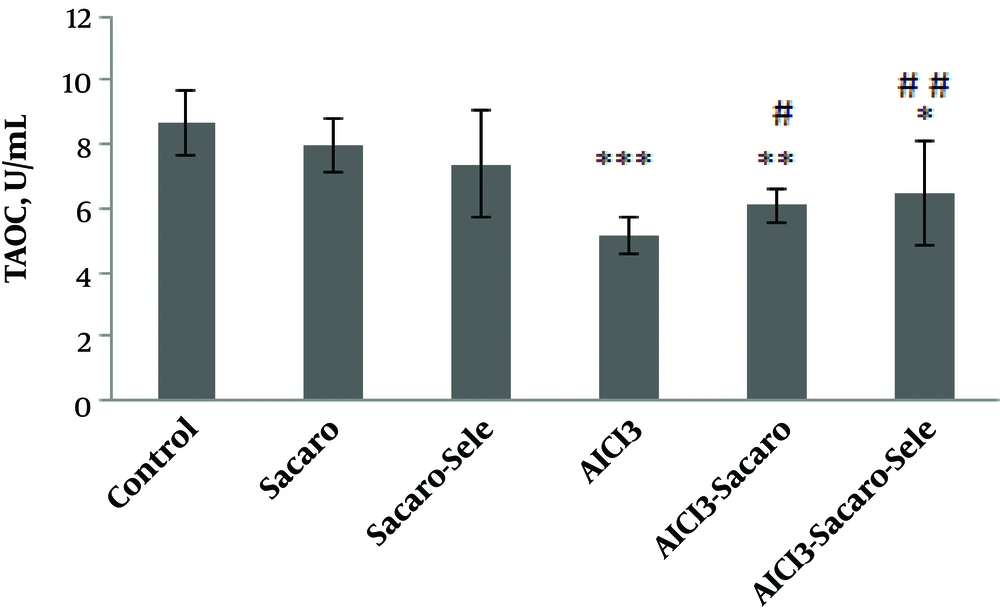
Also, as shown in Figure 3, T-AOC in the AlCl3-infected group, S. boulardii treated and AlCl3 infected group, and Se-enriched S. boulardii treated and AlCl3 infected group significantly declined as compared to the control group (P ≤ 0.001, P ≤ 0.01 and P ≤ 0.05, respectively).
Comparison of serum T-AOC concentrations between experimental groups. The results are expressed as mean ± SD. The asterisks indicate the significance levels of experimental groups as compared to the control group and # represents a comparison of the last two groups with the Al-infected group. Levels of significance values are *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, #P ≤ 0.05 ##P ≤ 0.01.
Significant increases of this parameter were seen in both infected groups treated with S. boulardii and Se-enriched S. boulardii as compared to the Al-infected group (P ≤ 0.05 and P ≤ 0.01, respectively).
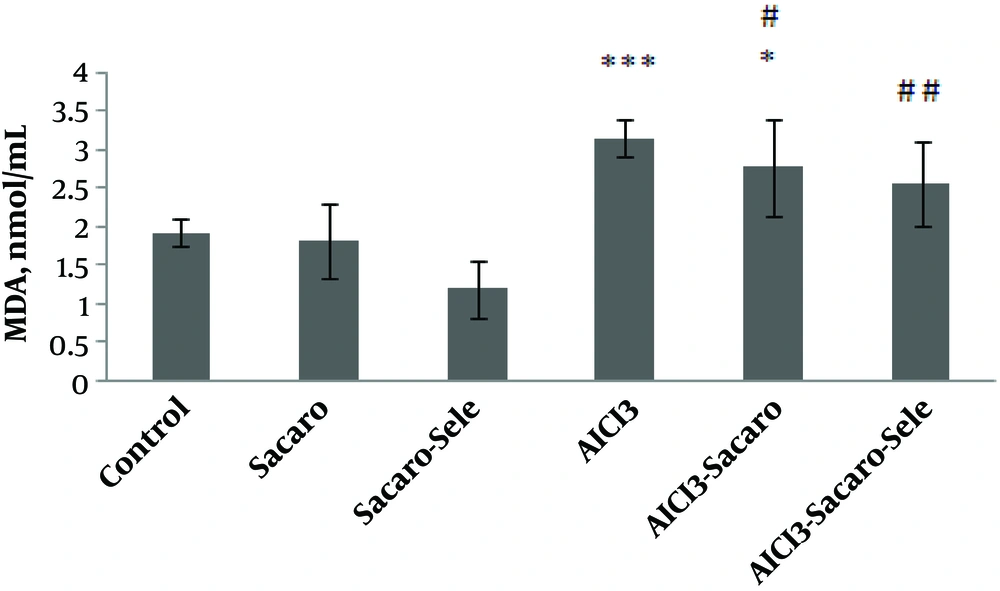
As shown in Figure 4, MDA in both the AlCl3-infected group and AlCl3 infected group treated with S. boulardii increased significantly as compared to the control group (P ≤ 0.001 and P ≤ 0.05, respectively).
Comparison of serum MDA concentrations between experimental groups. The results are expressed as mean ± SD. The asterisks indicate the significance levels of experimental groups as compared to the control group and # represents a comparison of the last two groups with the Al-infected group. Levels of significance values are *P ≤ 0.05, ***P ≤ 0.001, #P ≤ 0.05 ##P ≤ 0.01.
Malondialdehyde levels decreased in both infected groups treated with S. boulardii and Se-enriched S. boulardii as compared to the Al-infected group (P ≤ 0.05 and P ≤ 0.01, respectively).
4. Discussion
In the present research, the average number of platelets, RBC and its indices (MCH, MCHC), hemoglobin concentrations, and hematocrit percentages did not show any significant changes in the experimental groups as compared to the controls; whereas, the MCV (mean corpuscular volume) and RDW (red cell distribution width) in the Al infected group have shown significant decreases and increases, respectively. Changes in these two factors are directly related to the type of anemia. MCV reductions and RDW increases can be seen in heterogeneous microcytic anemia, which includes iron deficiency, S β-thalassemia, hemoglobin H, and red cell fragmentation (26). In a research conducted by Jacob and colleagues, increased blood lead levels apart from a slight increase in RBC led to reductions in some blood parameters such as MCV, especially in the girls (27). Bakour and colleagues reported significant reductions of MCV because of Al exposure (7). In the present study, MCV reductions in AlCl3-infected groups suggest that Al likely caused anemia. RDW changes above normal levels may indicate liver disease, anemia, and nutritional deficiencies. This parameter will increase in all types of anemia, including iron deficiency anemia; when its rates go up it implies anisocytosis (28). RDW rises in the AlCl3 exposed group increases the risk of anemia caused by Al. In both AlCl3-infected groups treated with S. boulardii and enriched S. boulardii, significant increases of MCV and decreases of RDW were found as compared to those in the AlCl3-infected group. We suggested that S. boulardii and enriched S. boulardii probably prevented anemia resulting from Al infection.
Continuing our study, iron and TIBC levels were also compared among the experimental groups due to the formation risk of iron deficiency anemia.
Iron concentrations decreased significantly in the AlCl3-infected group while it increased significantly in both S. boulardii and enriched S. boulardii treatment groups. Caramelo and colleagues declared that microcytic anemia caused by Al in dialysis patients with long-term treatment that microcytosis created by the Al was due to insufficient iron in most cases (29). In a study by Chmielnicka and colleagues, normocytic anemia was observed in mice poisoned with aluminum chloride. They found a direct relationship between the dose of metal and serum iron concentrations, blood parameters, and changes in the activity of enzymes involved in heme biosynthesis (21). Mahieu and colleagues (30), and Farina and colleagues (31), reported lower levels of serum iron in separate studies due to Al exposure. A reduction in serum iron levels due to Al exposure can be attributed to the effects of Al on iron movement at various levels, poor absorption of iron in the intestine, and interference in iron cellular absorption (29). Significant increases in iron concentrations in both treatment groups probably reflect the ability of S. boulardii and Se-enriched S. boulardii to produce vitamin B12 with probiotics (11) or an increased intestinal absorption of iron (10).
Serum iron levels in both infected groups treated with S. boulardii and enriched S. boulardii as compared to the AlCl3-infected group showed significant increases. Increased serum iron levels under the influence of S. boulardii, especially when enriched with Se, could have had a compensatory effect on serum iron reductions caused by Al.
Based on some available evidence, probiotics can promote iron absorption by creating an acid environment, convert ferric iron to ferrous iron, reduce phytase levels, as well as produce a variety of B vitamins, including vitamin B12 (32, 33). This is why a group of researchers has recommended the daily consumption of probiotic-containing diets for anemic people (10, 34).
Furthermore, despite insufficient studies on the impacts of Se-enriched yeast on anemia, it was clearly demonstrated that due to the performance of selenoproteins, Se causes a reduction of reactive oxygen species (ROS) levels indirectly and prevents the hemolysis of erythrocytes membrane lipids and proteins. Thus by maintaining the survival of these cells it prevents anemia (35).
TIBC, or total iron binding capacity, shows the maximum amount of iron that can be connected to transferrin. Increased TIBC is a symptom of iron deficiency anemia (36). TIBC concentrations increased in the AlCl3-infected group as compared to the control group. Significant TIBC increases along with serum iron decreases in the AlCl3-infected group confirm the risk of iron deficiency anemia in this group.
Although there were significant increases in TIBC in both AlCl3-infected groups treated with either S. boulardii or with Se-enriched S.boulardii, this increase was lower than that in the AlCl3-infected group.
Therefore, highly significant decreases in this factor were observed in both groups by comparing their TIBC levels with that of the AlCl3-infected group. So, it can be concluded that both S. boulardii and enriched S. boulardii prevented the adverse effects of Al on serum iron surfaces to some extent but not completely.
In the present research, WBC increased significantly in the AlCl3-infected group; however, it showed no significant differences in other experimental groups as compared to the control group. In line with our results, Kalaiselvi and colleagues showed that long exposures to Al could result in an increase in the number of WBC as compared to the control group (37). This suggested that WBC increases may be either associated to immune responses or protective reactions against oxidative stress induced by Al. In fact, lymphopoiesis stimulation or abundant releases of lymphocytes from lymphomyeloid tissues under the stress of toxicity may result in an increase in WBC. Mugahi and colleagues had observed leukocytosis after lead infection in rats and reported that it was due to increased numbers of lymphocytes, neutrophils, and monocytes (38).
Morsy and colleagues also found significant increases in white blood cells after the exposure of rats to aluminum oxide (AL2O3) (39).
It seems that increases in WBC are caused either by an inflammation in the body or the induction of oxidative stress by AlCl3.
A close connection exists between inflammation formation and induction of oxidative stress in the body-that is via immune system stimulation upon the entrance of foreign agents into the body; immune cells mobilize and secrete various inflammatory cytokines such as interleukin-6 and the tumor necrosis factor, which in turn leads to ROS release and then oxidative stress induction in the body (40, 41).
So, in this study, reduced amounts of white blood cells in both Al-infected groups treated with S. boulardii and enriched S. boulardii as compared to the infected group that was not treated can indicate the beneficial effects of this probiotic, especially in Se-enriched form, on modulating the host immune system function because of its antioxidant function.
Yu and colleagues reported that Lactobacillus plantarum CCFM639 exerts its protective effects on inflammation and Al-induced oxidative stress by preventing the activities of oxygen free radicals and inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), reducing MDA levels, and increasing the activities of SOD and CAT (42).
A group of researchers has shown that both probiotics and Se are able to prevent the induction of oxidative stress by eliminating oxygen free radicals and metal ions, and also protect the body against the adverse effects of heavy metals with their antioxidant properties (12, 43, 44).
It was mentioned earlier that oxidative stress induction is the symptom of heavy metals toxicity. Therefore, the T-AOC and MDA levels were compared between groups. In the AlCl3-infected group, significant reductions in the T-AOC levels and significant increases in MDA concentrations were observed which could be due to the oxidative stress induced by Al. In line with this consistency, in their research Sargazi and colleagues attributed kidney damage and increased MDA levels in Al toxicity to non-controlled production of free oxygen radicals in the cells and tissues (5). In another study, lipid peroxidation as well as reduced glutathione levels and activities were observed in the renal tissues of rats after long exposures to aluminum lactate (45). Increased levels of MDA caused by Al toxicity has been proved in other research (25, 46, 47). This is consistent with the results of the present study and confirms the induction of oxidative stress because MDA is a marker for lipid peroxidation measurement and oxidative stress evaluation (1). A team of researchers believes that Al generates large amounts of free oxygen radicals in processes like the Haber and Fenton processes, which in turn cause oxidative damages to the tissues and weaken the body’s antioxidant defense system by increasing the oxidation of important biomolecules and peroxidation of cell membranes (48-50).
In the present study, although decreased T-AOC and increased MDA levels were observed in both AlCl3-infected groups treated with S. boulardii or enriched S. boulardii, these changes were not as large as those of the AlCl3-infected group.
As compared to the Al-infected group in both S. boulardii and enriched S. boulardii treatment groups, MDA and T-AOC levels significantly decreased and increased, respectively. However, these changes were more significant in the S. boulardii treatment group. It seems that S. boulardii and selenium synergistically declined oxidative stress levels due to their antioxidant functions, but their impact was not strong enough to thoroughly overcome the induction of oxidative stress caused by Al toxicity.
Probiotics are able to scavenge free oxygen radicals, remove metal ions, inhibit oxidant compounds and prevent their production (12). Yu and colleagues reported that the antioxidant properties of probiotics, including Lactobacillus plantarum, could reduce oxidative stress caused by Al and thereby reduce its toxicity (1). In another study, by inhibiting oxidative stress through reducing free oxygen radicals and lipid peroxidation, Se supplements led to the reduction of kidney damage in rats which had been exposed to gentamicin (43). Ghorbel and colleagues reported in their study that Se supplements were not only able to reduce oxidative stress levels by restoring the antioxidant status in AlCl3-exposed mice, but they were also effective in the prevention of liver damage because of their antioxidant properties (19). In a similar study, El-Demerdash observed reduced levels of free radicals and cholesterol in addition to increased amounts of glutathione-S-transferase and total protein after taking selenium supplements in order to deal with Al toxicity (51).
Studies on the positive effects of Se-enriched probiotics as compared to probiotics alone are limited; but according to facts, both probiotic and selenium have antioxidant properties. It seems that a combination of these two factors had synergic effects on dealing with Al toxicity as compared to the consumption of probiotics alone. This was also proved in a comparative study by Shi and colleagues who showed that using organic Se supplements (yeast enriched with Se) in the diet of pregnant Taihang black goats had been more effective on their antioxidant status, hormonal secretions, and hematobiochemical parameters than consuming its mineral supplements (52).
According to conducted research, Se-enriched yeast possesses characteristics similar to those of selenomethionine which is essential for the body, but is not made by the body and must be supplied in dietary sources (53, 54). So, Se-enriched yeast can be used as a precursor for the synthesis of selenoproteins that are antioxidant enzymes and prevent cellular damages caused by the activities of free radicals (55).
4.1. Conclusions
Obtained results in this study indicated that due to its antioxidant properties and potential intestinal iron absorptions, S. boulardii prevents the decline of serum iron concentrations and oxidative stress induction caused by Al toxicity to some extent. Since Se is an essential element for the proper function of some antioxidant enzymes, using it with this probiotic may have had synergistic effects on the results. However, more studies, including increased treatment durations, doses, and analysis of other oxidative and anti-oxidative factors are needed to confirm the results.