1. Background
Leishmaniasis is a zoonotic disease that is observed all over the world, and presents as cutaneous leishmaniasis (CL), visceral leishmaniasis (VL) (Kala-Azar) and mucosal and dermal lesions.
The cause of leishmaniasis is a protozoan namely leishmania belonging to the order Kinetoplastida, which is observed in flagellated and non-flagellated forms depending on its living environment. This parasite lives and proliferates inside mononuclear phagocytes in vertebrates, and is generally transmitted by the bite of a species of sandfly, namely phlebotominae (1, 2).
Despite identifying the cause, carrier and ways of transmission and performing numerous studies on zoonotic cutaneous leishmaniasis (ZCL), this disease is still considered indigenous to many countries, and is even predicted to develop. This problem also exists in Iran due to the presence of the carriers and reservoirs of the disease almost everywhere in the country, and in recent years, the disease is emerging in many regions which were used to be free of the disease. Moreover, given the presence of the carriers in most parts of Iran, the disease is likely to become indigenous even to clean areas (3).
According to the WHO, leishmaniasis is indigenous to 98 countries, and more than 350 million people are at risk for developing the disease. About 12 million people are estimated to be infected with different types of leishmaniasis worldwide, and two million new cases to emerge annually, including 0.5 million with Kala-Azar and 1.5 million with CL. Moreover, Iran is among the 7 leading countries in terms of the incidence (4-6). Out of 22 Middle-Eastern countries, 14 countries are considered the focus of anthroponotic or urban ZCL caused by L. tropica and rural ZCL caused by L. major, including Afghanistan, Egypt, Iraq, Jordan, Libya, Morocco, Palestine, Pakistan, Saudi Arabia and Syria. The disease foci are also observed in many parts of Iran; rural type of ZCL is common in rural areas (more than 70% of infected cases) and the urban type in large and medium-size cities (7, 8).
According to the latest national report presented in a 2017 annual meeting on zoonotic diseases in Markazi province, Iran, over 16000 cases of ZCL have been recorded and reported in Iran in 2016 with a prevalence of 18.6 per 100,000, with Fars, Razavi Khorasan, Khuzestan, Isfahan, Ilam, Golestan, and Kerman being the most infected provinces involving more than 72% (12179) of the cases (9).
The distribution and spread of this disease are affected by socioeconomic and cultural factors, especially environmental and climatic conditions. Furthermore, global fighting with this disease has been turned into a challenge due to the complex cycle of propagation and diverse ecology of the disease and the effective factors in the habitat distribution of the reservoirs and carriers (10).
In the past two decades, the epidemiological pattern of ZCL has changed in Iran due to population movement between urban and rural areas and the influx of migrants from neighboring countries such as Afghanistan and Iraq. Socioeconomic and cultural factors are also involved in the prevalence of the disease (11).
According to the Iranian Center for Disease Control and Prevention, Iran was divided into four regions in terms of new cases of the disease, ranging from 0.2 to 180 cases in every 100,000 individuals, with Kermanshah being considered a moderate-incidence province with 13.4 cases per 100,000. Given the diverse climatic and ecological conditions of the province, a significant number of patients are annually diagnosed with ZCL in its tropical regions, especially in Qasr-e-Shirin, Sarpol-e-Zahab and Gilan-e-Gharb, which exhibit the disease focus and local transmissions (9).
Numerous studies have been conducted on ZCL in Kermanshah province, including the studies conducted by Hamzavi et al. in 2009 (12) and Hamzavi and Khademi in 2013 (13) on the epidemiological pattern of the patients in the province in 2001 - 2006 and 2013 respectively. A study conducted by Akia and Hamzavi (14) in 2016 on the molecular detection and determining the species of leishmanial parasite in patients with ZCL using the RFLP-PCR technique found 14.9% of the samples to be L. tropica-positive and 84.1% L. major-positive. In 2013, Yazdanpanah et al. investigated the effect of climatic factors on the prevalence of ZCL in Qasr-e-Shirin, and observed negative significant correlations between the mean temperature and the prevalence of ZCL (10).
2. Objectives
The present research was therefore performed to determine the effectiveness of leishmaniasis control programs in Qasr-e-Shirin, as the main disease focus in the province, by administering a comprehensive combined program, including fight against the carriers, fight against reservoirs and raising public awareness in 2015 - 2016. The obtained results were compared with the disease incidence two years before performing the intervention.
3. Methods
In the present quasi-experimental community trial, Qasr-e-Shirin was selected based on the disease incidence for implementing rural ZCL control programs in 2015 - 2016. After administering the program, the results obtained were compared with the disease incidence in 2013 and 2014 (baseline).
Hunting the rodent reservoir before the intervention revealed Tatera indica to be the disease reservoir. Entomological checks also confirmed the presence of sandflies in the region and activities of female sandflies in residential areas. A study conducted by Akia et al. in 2016 for determining the disease vector found L. major to be the main parasitic carrier in Qasr-e-Shirin. Interventional programs for controlling the disease were therefore carried out in Qasr-e-Shirin in three parts, including environmental improvements, trapping (using rodenticides) and spraying (using insecticides) and health education. The intervention scope included the city border where the nests of Tatera indica were observed at a 500-m radius from the most marginal residential building and villages with local transmissions of ZCL in 2013 - 2014.
The intervention proceeded with the start of sandflies’ activity in late April as follows: Destruction of rodents’ nests up to a 500-m radius around villages and the city border as previously described. The nests reopened by rodents as active nests were baited with wheat smeared with 2.5% zinc phosphide 48 hours later. A week later, reopened nests were rebaited with 2.5% zinc phosphide.
Deltamethrin WG 25% was used as insecticide per each 10 L Hudson water sprayer, covering 250 m2 of building surfaces. Environmental improvements comprised collecting debris from urban and rural areas, clearing the city and village from ruined buildings and the excavation and leveling of construction waste.
The operation of fighting with the reservoir in infested areas was performed four times a year in April, May, June and September by flattening rodent nests and baiting with wheat stained with 2.5% zinc phosphide. To control ZCL, the public were taught about the effect of residential building rehabilitations and installing nets and curtains on the entrance and windows on reducing the disease prevalence. Groups at risk for the disease were also trained by putting up banners, posters and writings in urban and rural public places. Moreover, pamphlets were distributed among primary school students, and educational classes were held on individual prevention and protection for the public and health workers (15).
After performing interventions in 2015 and 2016, and examining and completing epidemiological forms, the data obtained were analyzed in Minitab and compared with the data associated with 2013 - 2014 received from the Center for Management of Communicable Diseases in Kermanshah University of Medical Sciences. The Z-test was used to compare the disease incidence in Qasr-e-Shirin in 2013 - 2014 (before the intervention) with that in 2015 - 2016 (after the intervention).
4. Results
The data analysis of the disease frequency found a total of 162 patients developing the disease between 21 March 2013 and 20 March 2017 in Qasr-e-Shirin, including 96 (52%) males and 66 females. The disease was also observed in all age groups from 1 to 81 years. The highest frequency (80%) was observed in the active group of the community, i.e. 15 - 65 year olds.
Given the onset of activity of the mosquito carrying the disease, which heavily depends on regional climatic conditions, and the disease latency period, i.e. 3 months in rural types and 6 months in urban types, the disease was diagnosed in all seasons of the year. The highest incidence of the disease reported in this 4-year period was 40% associated with the end of the latency in winter.
In terms of the lesion site, open and unprotected parts of the body where the mosquito carrying the disease can easily bite were mostly involved. The data analysis found the wound in the hands and feet in 84% of cases. In terms of occupation, the patients’ were mostly housewives (31%), followed by farmers (29%) (Table 1).
| Variable | Before Intervention | After Intervention | Totala | ||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | Total | 2015 | 2016 | Total | ||
| Gender | |||||||
| Male | 22 | 39 | 61 | 22 | 13 | 35 | 96 (52) |
| Female | 15 | 23 | 38 | 23 | 5 | 28 | 66 (48) |
| Age, y | |||||||
| < 15 | 4 | 7 | 11 | 10 | 1 | 11 | 22 (13) |
| 15 - 65 | 31 | 32 | 63 | 50 | 16 | 66 | 129 (80) |
| over 65 | 2 | 5 | 7 | 3 | 1 | 4 | 11 (7) |
| Season | |||||||
| Spring | 12 | 6 | 18 | 9 | 1 | 10 | 28 (17) |
| Summer | 3 | 13 | 16 | 16 | 2 | 18 | 34 (21) |
| Autumn | 10 | 15 | 25 | 11 | 0 | 11 | 36 (22) |
| Winter | 12 | 28 | 40 | 9 | 15 | 24 | 64 (40) |
| Lesion site | |||||||
| Hand | 25 | 34 | 59 | 25 | 10 | 35 | 94 (58) |
| Foot | 7 | 19 | 26 | 25 | 10 | 35 | 42 (26) |
| Trunk | 0 | 3 | 3 | 4 | 0 | 4 | 7 (4) |
| Head and neck | 5 | 6 | 11 | 7 | 1 | 8 | 19 (12) |
| Occupation | |||||||
| Housewife | 11 | 18 | 29 | 18 | 4 | 22 | 51 (31) |
| Child and student | 2 | 1 | 3 | 1 | 3 | 4 | 7 (4) |
| Employee | 2 | 1 | 3 | 2 | 1 | 3 | 6 (4) |
| Farmer | 11 | 18 | 29 | 15 | 4 | 19 | 48 (29) |
| Army officer | 8 | 17 | 25 | 3 | 1 | 4 | 29 (18) |
| Others | 3 | 7 | 10 | 6 | 5 | 11 | 21 (14) |
aValues are expressed as No. (%).
The data analysis of the disease frequency showed reductions in the emergence of ZCL at different levels in Qasr-e-Shirin following the two-year intervention. The number of new cases reduced from 62 in 2014 to 45 in 2015 and 18 in 2016. The disease incidence also decreased from 14.5 cases in every 10,000 individuals in 2013 to 7 in every 10,000 in March 2017.
The Z-test (prevalence in previous years) showed significant differences in the incidence before and after administering the combined control program, suggesting a significant declining trend in this period (P < 0.05) (Table 2).
| Year | Frequency and Incidence of the Diseasea | |
|---|---|---|
| Frequency | Incidence | |
| 2013 | 37 | 14.5 |
| 2014 | 62 | 24.2 |
| Sum total associated with 2 years before the intervention | 99 | 38.3 |
| 2015 | 45 | 17.6 |
| 2016 | 18 | 7 |
| Sum total associated with 2 years of the intervention | 63 | 24.3 |
aP value < 0.05.
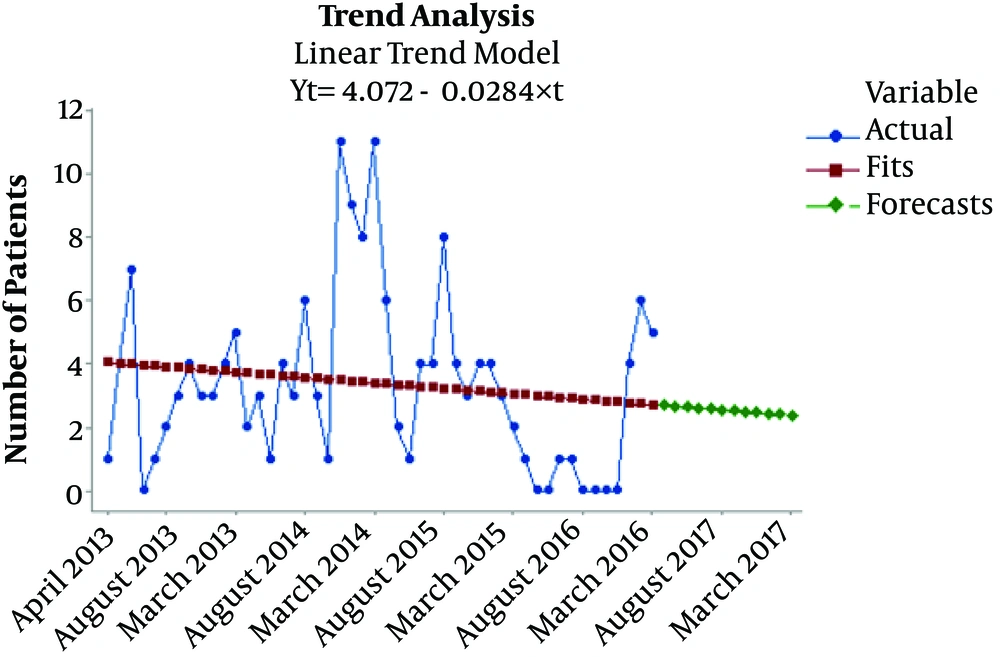
To accurately understand the incidence of ZCL in Qasr-e-Shirin, the cases were investigated in terms of three categories: first, the number of identified cases, second, statistical smoothing of the cases and statistical fit of previously accumulated cases diagnosed later and calculating the fit of the probabilistic trend of the emergence of the disease, and third, predicting the trend of emergence for the upcoming year based on the number of new cases observed over the previous four years. In case the combined intervention program is continued and the existing conditions remain unchanged, the four-year trend of the incidence of ZCL would be descending in Qasr-e-Shirin, and this downward trend is predicted to continue between March 2017 and March 2018 based on the current conditions (Figure 1).
5. Discussion
Research attributes a low endemicity to ZCL in Kermanshah province, and suggests that the disease is indigenous only to the western cities of the province where the weather is tropical, including Qasr-e-Shirin, Sarpol-e-Zahab and Gilan-e-Gharb (12, 16, 17). According to existing statistics, the number of new cases of the disease has been increasing from 1990 to 2016, reaching from 53 cases per year in 1990 - 1994 to 261 in 2016 in an oscillatory fashion. The frequency of the new cases is also ascending (16, 18).
Measures have been occasionally taken to control ZCL in Qasr-e-Shirin, which used to have the highest incidence in Kermanshah province in previous years. The effect of these interventional programs on reducing the frequency of this disease was, however, insignificant due to the incoherent nature of the programs; nevertheless, the present intervention caused significant reductions in the incidence of the disease by utilizing all control measures and modeling the interventions conducted in other provinces involved (13, 19).
According to the present and previous studies on ZCL, epidemiological and ecological analyses and the risk factors for the spread of the disease, administering combined control programs can be crucial for controlling this disease in endemic regions (10, 12, 20).
The present study found the major of the patients to be male, potentially due to the dress code or occupations exposing men to the disease carrier more than women, which is consistent with the study conducted by Hamzavi et al. in Kermanshah province in 2009 (12).
In terms occupation, housewives were the most affected group, with is consistent with the studies conducted by Jarahi et al. in Mashhad in 2015 and Saeed Firoozabadi and Karami in Qir and Karzin in 2017. In terms of incidence in different seasons, the highest number of detections were made in winter, which is consistent with studies the conducted by Yazdanpanah et al. in 2013 and Saeed et al. in Fars in 2016 (10, 12, 21, 22).
No combined interventional studies were conducted yet on fighting and controlling ZCL in Kermanshah province. The present intervention was the first measure designed and implemented in collaboration with Kermanshah University of Medical Sciences and Qasr-e-Shirin country administration and governorate for assessing the effect of this program on ZCL control and possibly administering it in other ZCL-indigenous cities in Kermanshah province. In Iran, different studies and interventions have been conducted in indigenous regions of the disease, including a study conducted by Saghafipour et al. in Qom in 2011 (20), Dehghani et al. in Yazd-Ardakan in 2003 (23), Mohammadi Azni et al. in Damghan (24) and a study by Nilforoushzadeh et al. in Isfahan (25), most of which suggested a successful control of the disease using combined methods.
The intervention performed by Saghafipour et al. in ten infected villages in Qom Province, Iran, in 2010 - 2011 comprised four steps, including using mosquito nets smeared with poison, distributing insect repellent pens, environmental improvements and health education. The intervention caused the disease incidence to reduce from 28.3 per 100,000 in 2009 to 17.4 per 100,000 in 2010 and 11.2 in 2011 in the infected villages (20).
In a study conducted by Dehghani et al. in Ardakan in 2003, the control program comprised two parts: Fighting the disease reservoir rodents by baiting their nests with poisoned baits at a radius of 2 km around the infected villages and indoor residual spraying for controlling sandflies using Propoxur in the spring and summer of 2000. After completing the program, the incidence reduced in Ahmadabad city from 22.8 per 10,000 in 1999 to 10.7 per 10,000 in 2000. In the Turkabad village affiliated to Ardakan, this figure reduced from 35 per 10,000 to 29.9 per 10,000 (23).
After conducting the present study and intervention, the disease incidence was found to be decreasing, reaching from 14.5 per 10,000 in 2013 to 7 per 10,000 in 2016. The statistical fit predicted that this declining trend would continue in 2017 in case the combined interventions persist.
Certain interventional studies on fighting ZCL have failed to produce the desired outcome and cause statistically significant reductions in the incidence, including a study by Nilforoushzadeh et al. in Imamzadeh-Agha-Aliabbas region, Isfahan province, Iran in 4 consecutive years starting from 2011 using five control methods, including spraying with Baygon, baiting with anticoagulant poisons, changing the region’s vegetation, environmental improvements and installing nets on doors and windows of residential buildings. No significant differences were observed in the incidence before and after the intervention; nevertheless, in 2009, Mohammadi Azni et al. could reduce the incidence of ZCL in Damghan, Iran from 555 per 100,000 in 2004 to 327 per 100,000 in 2005 and 153 per 100,000 in 2006 using a combined fighting method, suggesting an overall reduction in the incidence by a factor of 3.6 (24, 25).
5.1. Conclusion
The present research and all the other studies conducted in Iran and other countries show that fighting ZCL involves special epidemiological complications which require developed and consistent programs. Given the climatic conditions, type of the disease carrier and reservoir and type of soil and buildings texture in Qasr-e-Shirin and the affiliated villages, the combined fighting (against the reservoir and carrier and public education) reduced the disease incidence by March 2017 to one third of the incidence in 2014, suggesting that multilateral interventions are the most effective method of fighting ZCL, which is only implementable with the assistance received from beyond the county and in collaboration with other entities involved and in consultation with the program experts.