1. Background
Breast cancer is globally regarded as the second leading cause of death in females. As the most common therapies for this cancer, chemotherapy, radiotherapy and surgery affect both the cancer cells and the surrounding healthy cells (1). Researchers have always been seeking to find safe biological therapies to treat diseases. Given the effectiveness of probiotics in the immune system, numerous studies have evaluated the anti-cancer activity of this category of microorganisms. Saccharomyces boulardii is a probiotic yeast whose effect on cancer treatment has been tested. In-vitro studies showed that the compounds extracted from yeast cell walls, including glutathione and manna, could regulate the immune response in the body (2). In-vivo studies have also shown the role of probiotics in suppressing early neoplastic lesions and cancer tumors in mouse models (3). Probiotics exert their anti-cancer effects through preventing the conversion of procarcinogenic to carcinogenic, connecting and disabling mutagen compounds, inhibiting the growth of procarcinogenes bacteria and increasing immune system functions (3).
Apoptosis is adjusted by a group of proapoptotic and anti-apoptotic agents, including BAX and BAD proteins and BCL-2. The dysfunction of each factor can develop different diseases such as cancer. In the absence of trophic factors and under the stimulus of certain death-inducers such as the gamma ray, the ultraviolet ray, and toxic drugs, the non-phosphorylated form of the proapoptotic BAD protein moves towards mitochondria and harnesses its anti-apoptotic function by binding to the Bcl-x/Bcl-2 complex available in the membrane of mitochondria (4, 5). Disorders in the BAD protein expression activate BCL-2 that connects to the BAX protein, thus preventing cell death. The anti-apoptotic protein of BCL-2 and the proapoptotic protein of BAX play key roles in regulating apoptosis (6, 7).
The high incidence of BCL-2 increases the cell survival by harnessing apoptosis, whereas increasing the incidence of BAX accelerates the cell death (8, 9).
2. Objectives
The present study was conducted to model breast cancer using DMBA, and to evaluate the protective effects, hematologic factors and potential proapoptotic effects of the cell debris and supernatant of Saccharomyces boulardii on tumors in the breast.
3. Methods
3.1. Experimental Design
Sixty matures female Wistar rats weighing 150 ± 5 g were purchased from the animal’s nest of Falavarjan Branch of Islamic Azad University, Falavarjan, Iran. The rats were kept in 12:12 light-dark cycles at 22°C, and had free access to distilled water and sterilized food. They were randomly divided into four groups of 8 as follows:
3.1.1. Control Group
The rats were fed with water and food throughout the experimental period, and 0.5 mL of sesame oil was injected into the area around their left nipple at the beginning of the test.
3.1.2. Cancer Group
Injection of 0.5 mL of the carcinogen, including 5 mL of DMBA dissolved in sesame oil, into the left nipple (10).
3.1.3. Group Treated with the Cell Debris of Saccharomyces boulardii
Induction of breast cancer in the same way as in the cancer group followed by the daily intra-tumoral injection of 100 µL of the cell debris of Saccharomyces boulardii (1.5 × 108 cfu/mL) for 21 days beginning two weeks after the start of the test when the tumors sizes had exceeded 10 mm (11).
3.1.4. Group Treated the Supernatant of Saccharomyces boulardii
Induction of breast cancer and the intra-tumoral injection of 100 µL of the Saccharomycesboulardii supernatant two weeks after beginning the test.
3.2. Preparing the Cell Debris and Supernatant of Saccharomyces boulardii
Saccharomyces boulardii was purchased as capsules from a German company. To restore the yeast growth, the powder inside the capsules were transferred to Mueller Hinton Broth, and incubated for 19 hours. The specimens were then cultured on Sabouraud dextrose agar using the linear method, and incubated for 48 hours at 37°C (11). Some physiology serum was added to the medium after the yeast growth. The door of the plate was closed, and it was shaken wavily until yeast latex was obtained and collected in sterile flasks when preparing the cell debris. A dilution containing 107 - 108 yeasts per mL was prepared using Neubauer slides. The latex was centrifuged at 5000 rpm for 10 minutes to prepare the supernatant, and the resulting supernatant was collected and kept in a refrigerator before it is used.
3.3. Tumor Volume Measurement
After inducing the cancer, the minimum (d) and maximum diameter (D) of the tumors were measured in groups B, C and D two, four and six after beginning the test. The volume of the tumors was then calculated using V = 0.5 × d2 × D (10).
3.4. Hematological Factors Measurement
After the treatment period, the animals were directly anesthetized through their hearts with diethyl ether and venipuncture. Then WBC, RBC, HGB, HCT, MCV, MPV, MCH, RDW, PLT, MPV, and PDW were measured with an automatic hematology device (XE-5000 Sysmex Corporation, Kobe, Japan).
3.5. Histopathologic Examinations
After collecting the blood samples, the rats were slaughtered with cervical dislocation. The tumors and normal breast tissues were completely removed and washed with phosphate buffered saline. One third of the tumors were placed in 10% formalin for 24 hours. Sections of the tumors were obtained and processed using a tissue processor through dehydration with ascending grades of alcohol, clearing, impregnation in paraffin and sectioning in molten paraffin. After the tissue processing, 5-µm cuttings of the samples were stained with hematoxylin eosin stain. An optical microscope was ultimately used to examine the histological changes (10).
3.6. BAX and BCL-2 Concentrations
One mg of the tumor in each rat was cut and homogenized with a phosphate buffer (pH 4.7). A certain volume of phosphate buffered saline was then added to each sample. The samples were centrifuged at 2000 - 3000 rpm for 20 minutes with a refrigerated centrifuge. The supernatants were carefully removed from the samples. The BAX and BCL-2 concentrations of the samples were determined according to the kit protocol, in a way that five concentrations of the standard solution were prepared, followed by adding HRP Streptavidin. Chromogens A and B and stop solution were then added to the blank wells. To test the wells, the samples, the BAX antibody and HRP Streptavidin were added and incubated at 37°C for 60 minutes. The chromogenic solutions A and B were respectively added to each well and incubated for 10 minutes at 37°C. The light absorbance of the samples was measured at 450 nm using an ELISA reader after adding the stop solution. The BAX concentration of the samples was calculated according to the standard curve plotted. The methods of measuring BCL-2 and BAX concentrations are generally the same except for the antibodies used.
3.7. Statistical Analysis
The paired t-test was used to analyze the experimental data associated with the tumor sizes and describe them as mean ± SD in SPSS-16, and one-way ANOVA for analyzing the other data obtained. P < 0.05 was set as the level of statistical significance.
4. Results
4.1. Evaluating the Tumor Volume in the Experimental Groups
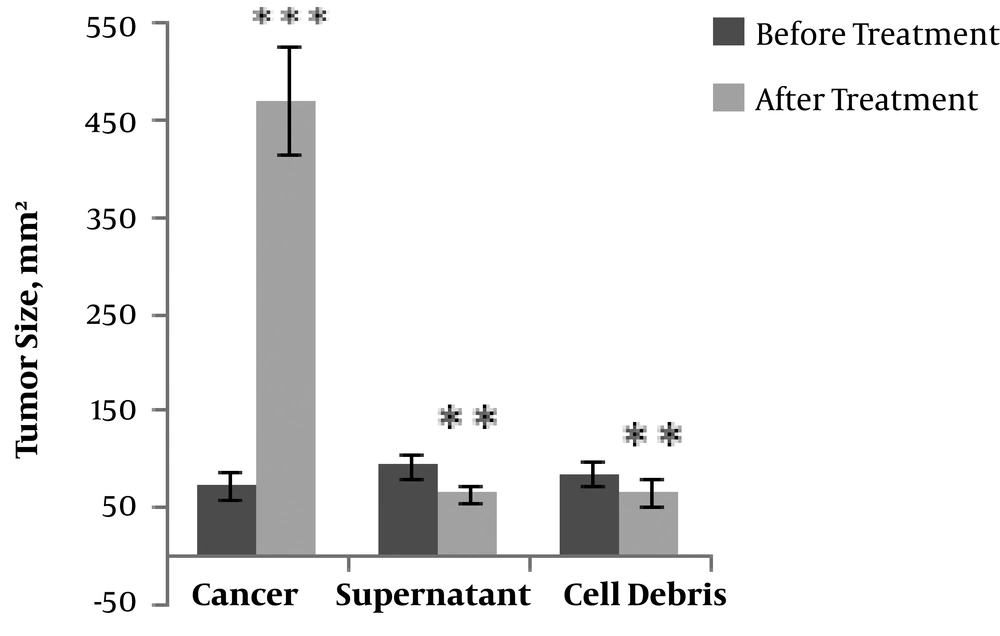
According to the data presented in Figure 1, the average volume of the tumors increased with time in all groups of the tested rats. Significant decreases were, however, observed in the tumor volume in the groups treated with either the cell debris (P < 0.01) or the supernatant (P < 0.001) compared to in the cancer group.
4.2. Comparative Study of the Hematological Parameters in the Experimental Groups
The number of neutrophils, WBCs and lymphocytes in the cancer group significantly increased compared to in the control group (Table 1). No significant differences were, however, observed in the groups treated with the cell debris or the supernatant compared to in the control group.
| Hematological Factor | Experimental Group | |||
|---|---|---|---|---|
| Control Group | Cancer Group | Treatment with Cell Debris | Treatment with Supernatant | |
| WBC, 103/µL | 7.2 ± 2.46 | 14.04 ± 1.44b | 8.14 ± 2.04 | 7.8 ± 1.64 |
| Neu, % | 31 ± 6.6 | 47.83 ± 6.61b | 25.5 ± 2.81 | 29.5 ± 5.28 |
| Lym, % | 7.16 ± 6.17 | 33.83 ± 9.52b | 71.5 ± 10.78 | 68.16 ± 9.92 |
| RBC, 106/µL | 6.91 ± 0.67 | 5.78 ± 1.03c | 7.05 ± 0.12 | 7.32 ± 0.39 |
| HGB, g/dL | 13.18 ± 1.06 | 11.13 ± 1.21d | 13.16 ± 0.82 | 13.38 ± 0.36 |
| HCT, % | 44.55 ± 4.93 | 34.63 ± 2.10b | 44.96 ± 2.17 | 43.78 ± 2.07 |
| MCV, fl | 64.38 ± 2.48 | 49.60 ± 1.45b | 60.86 ± 2.58c | 59.81 ± 1.87d |
| MCH, pg | 19.1 ± 0.58 | 17.83 ± 0.95c | 18.31 ± 0.38 | 18.30 ± 0.67 |
| RDWsd, fl | 41.81 ± 11.16 | 26.21 ± 1.25b | 35.81 ± 2.65 | 35.08 ± 2.74 |
| PLT, 103/µL | 7.27 ± 140.34 | 9.99 ± 70/11d | 8.52 ± 165.08 | 8.32 ± 42.09 |
| MPV, fl | 7.21 ± 0.72 | 5.81 ± 0.16b | 7.01 ± 0.21 | 6.70 ± 0.22 |
| PDW, fl | 15 ± 0.209 | 14.68 ± 0.147 | 14.93 ± 0.177 | 14.98 ± 0.271 |
Comparative Study of the Hematological Parameters in the Experimental Groupsa
The mean values of RBC, HGB, HCT, RDW, MCV and MCH significantly decreased in the cancer group compared to in the control group. Although the reduction in mean MCV observed in the groups treated with the cell debris or the supernatant of Saccharomyces boulardii was significant compared to in the control group, no significant differences were observed in the mean values of RBC, HGB, HCT, MCH and RDW in these groups compared to in the control group.
The number of platelets significantly increased and that of MPV significantly decreased in the cancer group compared to in the control group. Moreover, compared to in the control group, no changes were observed in PDW in the cancer group and in MPV, PDW and PLT in the groups treated with either the cell debris or the supernatant.
4.3. The Results of Histopathological Study
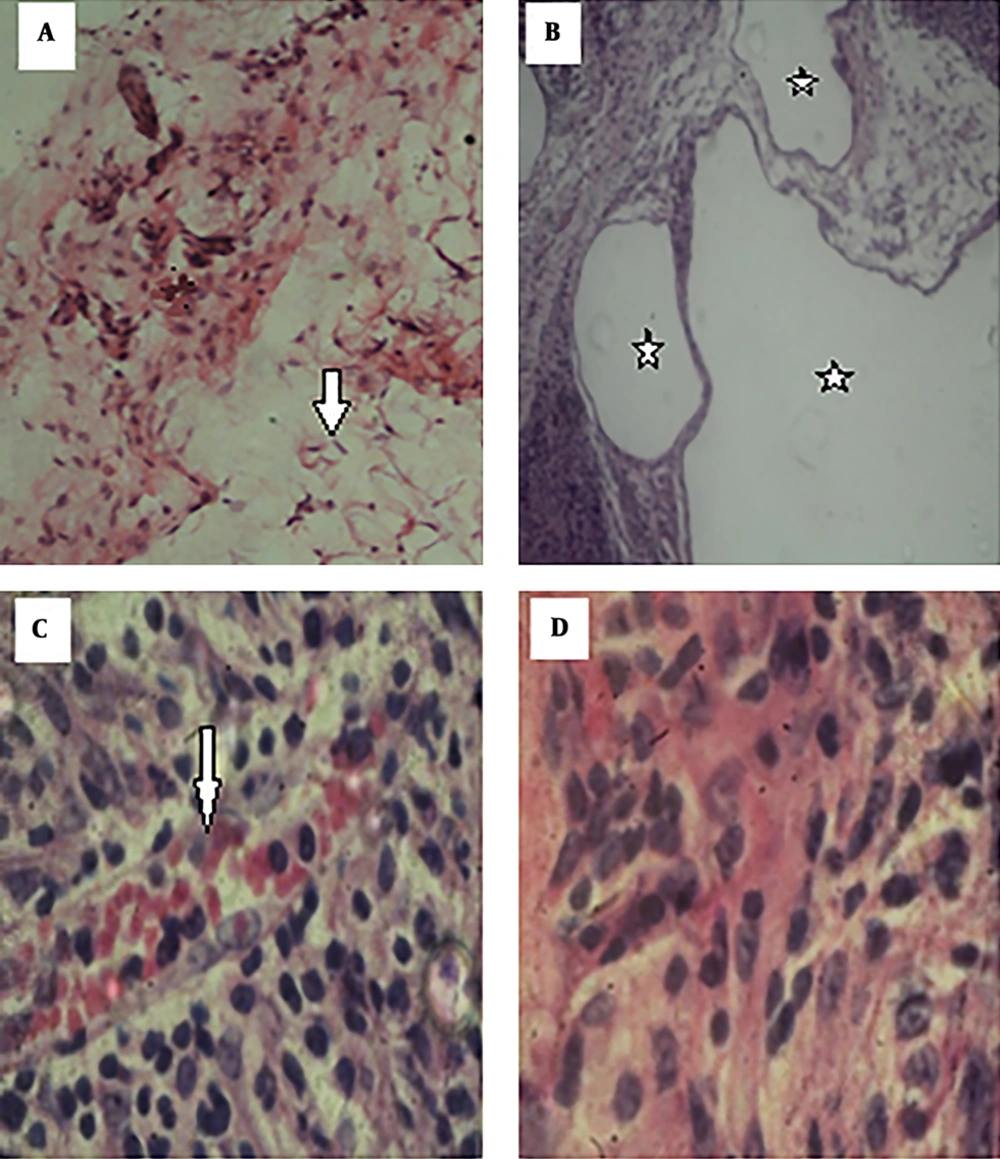
Hematoxylin and eosin staining showed strumal cells in the normal breast adipose tissue in the control group (Figure 2A). Changes were obvious in the breast tissue in the cancer group, as the lobules were not separated and the appearance of the tissue (acinis and ducts) suggested cancerous changes. The large size of the milk ducts also confirmed the duct carcinoma. In addition, deformation of the milk acinis suggested abnormal milk synthesis (Figure 2B).
Histopathological changes in the breast tissue. A, normal adipose tissue (arrows) in the control group (×100); B, hyperplasia and cancerous milk ducts (stars) in the cancer group (×40); C, aggregation of RBCs (arrows) between the breast cells in the group treated with the cell debris of Saccharomyces boulardii (×400); D, absence of histopathological changes in the group treated with the supernatant of Saccharomyces boulardii (×400).
The tissue slides in the cancer group treated with the cell debris of Saccharomyces boulardii suggested that Saccharomyces boulardii eliminated certain symptoms of the pathological tissue cancer while the appearance of the tissues was still inflamed and deformed (Figure 2C).
The tissue slides in the cancer group treated with the supernatant of Saccharomyces boulardii showed that although the acinis and milk ducts exited from the cancerous mode, the cancer tissue retained its abnormal form (Figure 2D).
4.4. Evaluating the Statistics and Results of BAX and BCL-2 Concentrations in the Tumors
The average concentration of BCL-2 significantly decreased in the group treated with the supernatant of Saccharomyces boulardii compared to in the cancer group, although this reduction was insignificant in the group treated with the cell debris of Saccharomyces boulardii.
Moreover, the average concentration of BAX significantly increased in the group treated with the supernatant of Saccharomyces boulardii compared to in the cancer group, while this increase was insignificant in the group treated with the cell debris (Table 2).
Comparing BAX and BCL-2 Concentrations in the Treatment Groups Compared to in the Cancer Group
5. Discussion
The present research investigated the effects of the cell debris and supernatant of Saccharomyces boulardii on DMBA-induced breast cancer in rats. The tumor sizes significantly decreased in both groups of treatment with the cell debris and the yeast supernatant compared to before the treatment. In line with the present study, the tumor volume was found to decrease in mice receiving Lactobacillus casei and Lactobacillus acidophilus compared to in the controls (12). The cell walls of Saccharomyces boulardii and Saccharomyces cerevisiae were also found to inhibit the growth of the cancer K562 (13), and the effect of the cell walls of Saccharomyces boulardii to be more significant than that of the cell walls of Saccharomyces cerevisiae. As a probiotic, Saccharomyces boulardii appears to moderate the deleterious effects of the cancer potentially by increasing the level of the body immune system, possessing a series of tumor growth inhibitors, and as a result of the presence of Beta-Glucan and Mannan in the cell wall (14). The present study found the cell debris and supernatant of Saccharomyces boulardii to reduce the level of cancer-associated inflammation, and to prevent an increase in WBCs, lymphocytes and neutrophils, while in the cancer group, the number of WBCs, lymphocytes and neutrophils increased due to inducing the cancer with the stimulation of the bone marrow. The studies conducted on hematological parameters in a population of cancer patients in Iraq found breast cancer to dramatically increase the number of WBCs and lymphocytes, and the cancer-associated inflammation in the body and the activation of immune cells to increase WBCs (15). In 2016, Mantas et al. reported significant increases in WBCs, lymphocytes and other inflammatory factors in women with breast cancer (16). The present study showed a decline in RBCs, hemoglobin, hematocrit, MCV and MCH in the cancer group compared to in the controls, suggesting anemia caused by inducing the cancer. The post-treatment modification of the cited factors with the cell debris and supernatant of Saccharomyces boulardii reflects the effectiveness of probiotics in strengthening immune system responses and preventing inflammations.
The significant decreases observed in RBCs, hemoglobin and hematocrit in the mice with breast cancer were associated with a kind of anemia, known as microcytic hypochromic anemia caused by increasing MCV. Different cytokines, including tumor necrosis factor, interleukin-1 and interferon gamma, involved in inflammatory processes contribute to developing anemia (17).
The present study observed an increase in platelets in the cancer group, which suggests the thrombocytosis induction. This increase was controlled using the cell debris and supernatant of Saccharomyces boulardii. This probiotic must have prevented the thrombocytosis induction by inhibiting the changes in the volume and shape of platelets.
In addition, the increase observed in the tumor volume and the unusual cell proliferation in the milk ducts suggest the induction of ductal carcinoma in the cancer group. The pathological and inflammatory symptoms of the cancer tissue significantly decreased after injecting the cell debris and supernatant of Saccharomyces boulardii potentially through the necrosis of the cancer cells, the inhibition of cellular mitosis and increasing anti-tumor responses. The accumulation of RBCs in the tissue sections in the group treated with the supernatant of Saccharomyces boulardii was less than that in the group treated with the cell debris of Saccharomyces boulardii potentially due to the inhibition of angiogenesis or bleeding following the cancer progression caused by the extract. In (18), the reduction observed in nucleus mitotic active forms and cellular necrosis after injecting cisplatin combined with Lactobacillus rhamnosus was more than that in the group receiving only cisplatin. The death of tumor cells was also observed in breast cancer groups treated with Lactobacillus reuteri according to the histological results of cancer tissues (19).
According to the present results, probiotics caused cell death probably by affecting different cancer cells. Further studies are therefore recommended to be conducted on the possibility of apoptosis induction with Saccharomyces boulardii. Furthermore, the increasing BCL-2 concentration observed in the cancer group was potentially associated with the over expression of the BCL-2 gene and mitochondrial membrane stability, whereas the decreasing BAX concentration in this group was potentially associated with the decreased expression of this protein. The BCL-2 reduction observed in the groups treated with the cell debris and supernatant of Saccharomyces boulardii was potentially associated with a decrease in the BCL-2 gene expression and the permeability of the mitochondrial membrane. The increase observed in the BAX concentration in the treatment groups was also associated with the increase in the BAX gene expression, activation of the pathways of mitochondrial cell death and the prevention of cell division (8). A study on 101 patients with breast ductal carcinoma reported an increase in BCL-2 in lower levels of breast malignancies, and found BCL-2 to be a determinant of cancer induction (20). The studies conducted on gene expression associated with programmed cell death in ovarian cancer cells treated with cisplatin showed that the expression of the Bcl-2 gene was decreased by the treatment with cisplatin, and that the BAX expression remained unchanged (21). Research suggests that the presence of compounds such as ergosterol and β glucan in yeast changes the factors associated with the apoptosis and apoptosis induction in cancer cells (13, 14).
The cell escape from the apoptosis is a mechanism contributing to cancer development. During this process, the non-phosphorylated forms of the proapoptotic BAD protein move toward mitochondria and bond with the Bcl-x/Bcl-2 complex available in the mitochondrial membrane, and inhibit its anti-apoptotic function in the absence of tropic factors and the presence of death-inducing stimuli, including gamma rays, ultraviolet radiation, and treatment with toxic drugs. In fact, the BAD connection prevents the reaction of the anti-apoptosis protein of BAX. As a proapoptotic protein, BAX bonds with the mitochondrial membrane, and provides channels for the flow of ions into the mitochondria in homodimer states. This ion flow resulted in the release of cytochrome C through the internal and external mitochondrial membranes into the cytosol. Cytochrome C activate a cascade of caspase, and causes the cell death (20, 21). Any factor potentially stimulating apoptosis can be considered a good candidate for cancer treatment.
5.1. Conclusions
According to the present study results, as a probiotic, Saccharomyces boulardii, especially the cytoplasmic extract with its numerous cytotoxic metabolites, has potential anti-cancer properties that prevent the growth of tumors through the induction of apoptosis in cells without exerting any adverse effects on hematological factors. Further studies are recommended to be conducted on other cancers and other factors involved in the apoptosis to determine the potential of this yeast to be applied as an appropriate candidate for cancer therapy. In addition, the potential apoptosis induction by this probiotic inferred from the reductions observed in BCL-2 and BAX concentrations in the cancer treatment groups requires further research.