1. Background
Sedentary lifestyle is responsible for over one-third of sudden deaths caused by cardiovascular diseases, cancer, and diabetes. Cardiovascular diseases are the main cause of mortality and morbidity in most industrial and developed countries, and together with cerebrovascular diseases, account for 40% to 50% of all deaths in these countries (1).
The role of exercise in the prevention, management, and treatment of cardiovascular diseases has been well demonstrated in several studies (2). The European Heart Association (2012) and the American College of Cardiology (2013) have recommended regular exercise for the prevention, treatment, and management of heart diseases, even for cardiac patients (3). Some recent studies have proposed that biomarkers of the heart muscle damage increase after exercise, even in healthy individuals (4), such that the serum levels of cardiac troponin T and iso-creatine kinase (which are the cardiac damage markers) rise as a result of exercise. Muscle damage in healthy people can be attributed to production of free radicals after intense training (5). Myoglobin and creatine kinase in muscle cells increase in men and women after prolonged exercise, and this increase is a sign of musculoskeletal damage (5). Furthermore, intense physical activities can potentially harm cardiac function (6), which is referred to as exercise-induced cardiac fatigue (7), and has recently been recognized by researchers as the least post-exercise cardiac cell damage (7, 8). New markers are now used in assessing heart muscle cell damage, namely: cardiac troponin I (cTn-I), cardiac troponin T (cTn-T) and iso-creatine kinase MB (CK-MB). Cardiac troponin T and I are the proteins in actin filaments that regulate the speed and force of contraction in cardiac cells. These are highly specific and sensitive markers for cardiac cell damage, and are extensively released into blood plasma following cardiac damage or fatigue (9, 10). CTn-T, cTn-I, creatine kinase (CK) and its iso-enzyme (CK-MB) are appropriate tools for diagnosis of damage to cardiac muscle cells, ischemia, and cardiac muscle infarction (11). Savukoski et al. assessed cardiac troponin after recreational resistance exercises, and reported a significantly higher concentration of cardiac troponin after exercise compared to before, and troponin returned to its original level after three days (12). Eijsvogels et al. reported increased levels of cTnI in individuals with normal weight following one session of moderate intensity activity (13). Legaz-Arrese et al. reported increased level of cTnI after a short high-intensity activity in professional and amateur oarsmen (14). The results obtained by Rajaei et al. showed that despite an increase in CK-MB, which may have been due to the nature of exercise and muscle damage because of intense activity, performing resistance, endurance, and combined exercises have no significant effect on cTnT level in active men, and therefore cannot cause cardiac damage (15). Despite several results showing the relationship between physical activity and reduced cardiovascular damage following regular exercise, conflicting information exists on the effect of resistance training on cardiovascular system. Furthermore, there are few studies on non-athlete women and high intensity resistance training. The present study is expected to partly clarify the mechanisms responsible for the changes in biochemical markers following resistance training, and lead to the identification of an appropriate intensity to gain greater benefit from resistance training.
2. Objectives
The present study aims to investigate the effect of acute resistance training on biochemical markers of myocardial damage (cTnI, cTnT, and CK-MB) in non-athlete women.
3. Methods
The study population consisted of all female students of Islamic Azad University in Baneh, Iran. Then, a notice for participation in the study was distributed in the university, and out of interested and eligible students, eighteen 19 - 26 year-old non-athlete female students were selected by purposive method. The inclusion criteria were no regular resistance training in the last six months, no acute or chronic diseases, no regular use of medications or amino acids, no use of cigarettes, and no blood or liver problems. The study objective and method and researcher’s expectations were explained and the subjects completed informed consent forms. The subjects’ age and medical/athletic history were assessed using a questionnaire. The subjects were asked to attend Mehran fitness club a week before the main test, where their height, BMI and body composition were measured and recorded. Measurements were carried out in the same conditions between 8 a.m. and 11 a.m. and at a predetermined time for each person. Then, participants were familiarized with the training hall, and trained on their correct use. They were also asked to fast for 10 - 12 hours the night before the blood sampling. The tests were carried out in the same place for all participants. One repeat maximum (1RM) test of all movements was determined for all participants. Height was measured using a stadiometer (precision of 0.1 cm), and weight with minimal clothing using Camry scales (EB9000, China) (precision of 0.1 kg). The percentage of body fat was determined using In-Body 3.0 (Korea) by electrical impedance method.
A week after determining 1RM, the subjects took part in a session of resistance exercise, which included chest press, leg press, shoulder press, biceps curls, knee flexion, cable lat pushdown and triceps cable pushdown. Each movement was performed in three sets and 10 repeats at 70% of 1RM, with two minutes resting period between sets and movements. Each test session lasted about 55 minutes (16). All subjects were tested in two successive sessions. Troponin T and I were measured using a third generation kit (Roche Company, Germany) by ELISA method. CK-MB was measured by CK activity kit (Roche Company, Germany).
Samples of 3 mL venous blood were taken from participants’ brachial vein under the same conditions on five time points: before training program, immediately, 1h, 24 hours and 48 hours after training. The samples were sent to Hakim private laboratory, where they were centrifuged at 2700 rpm for 15 minutes, and the serum was partly transferred to test tubes.
Data were analyzed using inferential statistical tests, including repeated measures ANOVA, and if required post hoc Bonferroni test. The pretest and posttest results were compared using t-test. The results were analyzed at P ≤ 0.05. All analyses were carried out in SPSS V. 18, and graphs were plotted in Excel.
4. Results
Mean and standard deviation of participants’ descriptive details are presented in Tables 1 and 2.
| Variable | Valuesa |
|---|---|
| Age, y | 23.41 ± 1.49 |
| Height, cm | 162.09 ± 5.08 |
| Weight, kg | 57.40 ± 3.73 |
| BMI, kg/m2 | 21.62 ± 0.50 |
Participants’ Descriptive Details
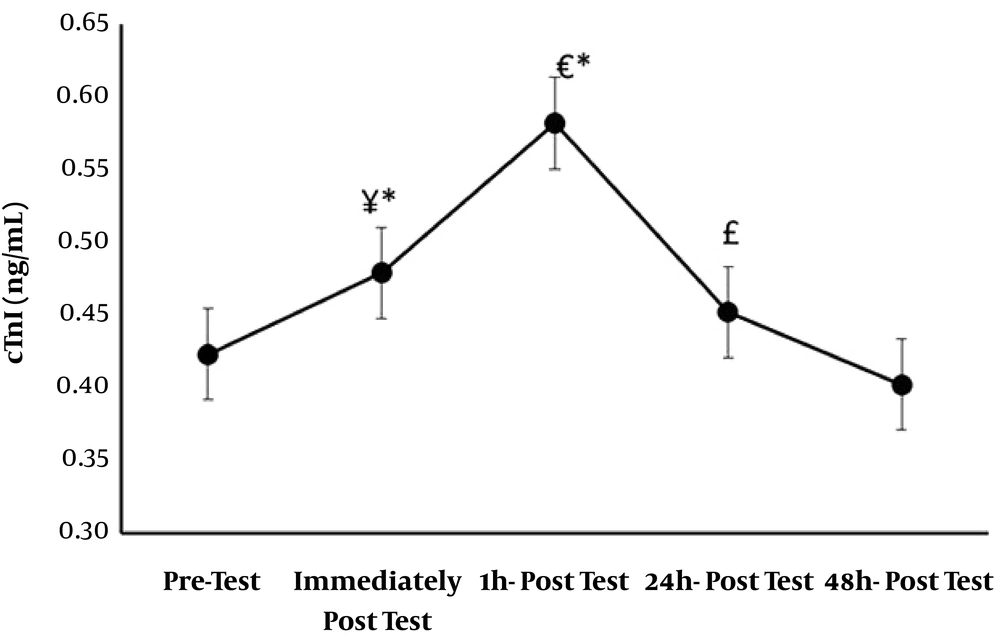
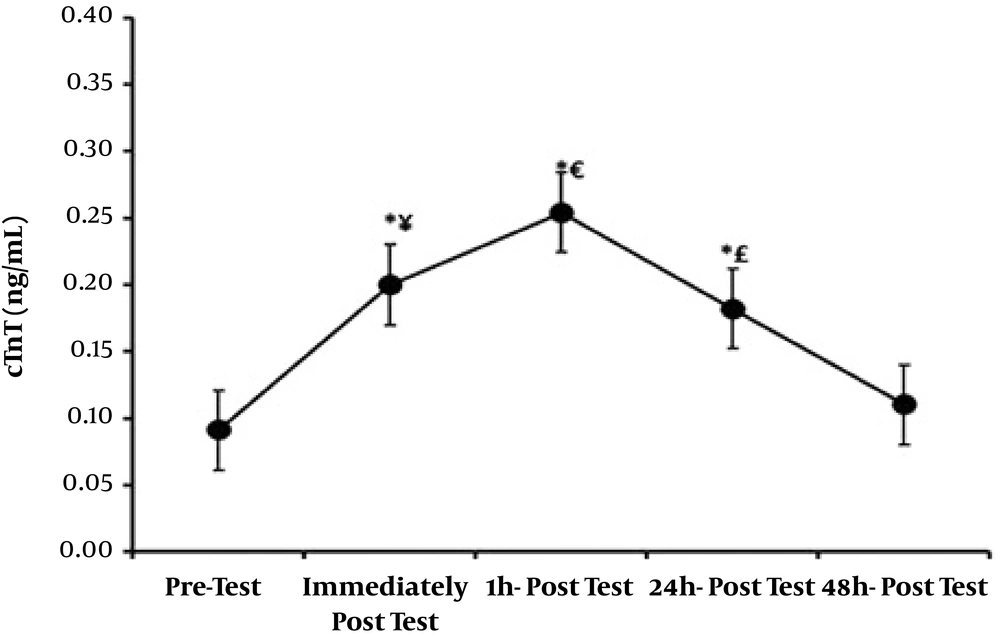
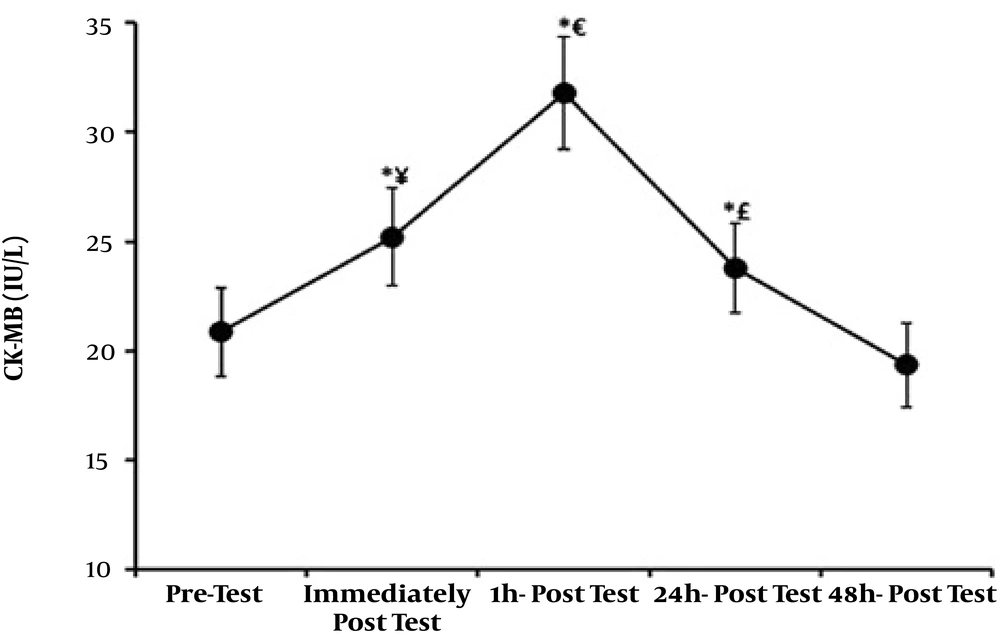
| Troponin I (ng/mL) | Troponin T (ng/mL) | Creatine Phosphokinase (IU/L) | |
|---|---|---|---|
| Before training | 0.423 ± 0.055 | 0.091 ± 0.0021 | 20.85 ± 2.03 |
| Immediate after training | 0.479 ± 0.046 | 0.20 ± 0.0018 | 25.20 ± 2.23 |
| One hour after training | 0.582 ± 0.063 | 0.254 ± 0.0011 | 31.80 ± 2.58 |
| 24 hours after training | 0.452 ± 0.047 | 0.182 ± 0.0015 | 23.80 ± 2.06 |
| 48 hours after training | 0.402 ± 0.050 | 0.11 ± 0.0019 | 19.35 ± 1.92 |
Mean and Standard Deviation of the Study Variablesa
Normal distribution of data was confirmed by Kolmogorov-Smirnov test, and therefore, parametric tests were used to test the study hypotheses. The results showed that cardiac troponin I significantly increased immediately and one hour after resistance training (P < 0.01), but no significant increase was observed 24 hours and 48 hours after resistance training and troponin I values returned to baseline. Significant increases were also observed immediately (P < 0.01), one hour (P < 0.01), and 24 hours (P < 0.04) after resistance training in troponin T values and CK-MB (P < 0.01), but troponin T and CK-MB values returned to baseline 48 hours after resistance training. Significant differences were observed in troponin I, troponin T, and CK-MB values between immediately after and 1, 24, and 48 hours after resistance training; between one hour after and 24 and 48 hours after; and between 24 hours after and 48 hours after resistance training. Figures 1 - 3.
ANOVA test results comparing participants’ troponin I values on different occasions. *, Shows a significant difference compared to pretest (P < 0.01); ¥, Shows a significant difference between immediately after and 1, 24, and 48 hours after resistance training (P < 0.01), (P < 0.03), (P < 0.01); €, Shows a significant difference between one hour and 24 and 48 hours after resistance training (P < 0.01); £, Shows a significant difference between 24 hours and 48 hours after resistance training (P < 0.02).
Repeated measures ANOVA results comparing participants’ troponin T values on different occasions. *, Shows a significant difference compared to pretest (P < 0.01); ¥, Shows a significant difference between immediately after and 1, 24, and 48 hours after resistance training (P < 0.01), (P < 0.04), (P < 0.01); €, Shows a significant difference between one hour and 24 and 48 hours after resistance training (P < 0.01); £, Shows a significant difference between 24 hours and 48 hours after resistance training (P < 0.01).
Repeated measures ANOVA results comparing participants’ creatine phosphokinase values on different occasions; *, Shows a significant difference compared to pretest (P < 0.001); ¥, Shows a significant difference between immediately after and 1, 24, and 48 hours after resistance training (P < 0.001); €, Shows a significant difference between one hour and 24 and 48 hours after resistance training (P < 0.002); £, Shows a significant difference between 24 hours and 48 hours after resistance training (P < 0.001).
5. Discussion
The present study aimed to investigate the effect of acute resistance training on biochemical markers of myocardial damage (cTnI, cTnT, CK-MB) in non-athlete female students. ANOVA test results showed that acute resistance training significantly increased cTnT immediately, 1 hour and 24 hours after training, and cTnI immediately and 1 hour after training in non-athlete female students. The cTnT values returned to baseline after 48 hours, which agrees with the results obtained by Tian et al., Shimizu et al., Koniq et al., Lowbeer et al., Shave et al., Tanga et al., and Legaz et al. (12, 14, 17-21). Also, no changes in cTnI 24 hours and 48 hours after training concur with studies conducted by Karimjee et al., Ashmaig et al., and Kolleer et al. (22-24). However, the present study results disagree with those obtained by Rajaei et al., Konig et al., van der Linden et al., and Chen et al., which reported that one session of training has no effect on troponin value (15, 25-27).
An increase in cardiac troponin values has been observed in athletes following acute training. Since an increase in troponin released in blood is indicative of acute cardiac muscle damage (except in patients with kidney diseases), it can be inferred that training can lead to increased risk of heart failure. It should be noted that despite a significant increase in troponin values, the increase was far less than the diagnostic reference limit for the risk of heart failure (19). In the present study, troponin values decreased after 24 and 48 hours, while the incidence of heart failure has been reported 24 hours after the increase and troponin value peaks after 72 hours (19). Many theories have been proposed about the reasons behind the increase in cardiac troponin after training. Exercise training increases permeability of myocardial sarcolemma, which probably facilitates the release of cytosolic cardiac troponin. Hence, after training, cardiac troponin is probably released to the extracellular space by passive diffusion. Increased permeability of the membrane is probably due to the increase in mechanical stress on myocytes, increased synthesis of oxidative radicals, or alkaline-acid imbalance (21). Jassal et al. concluded that the increase in cardiac damage markers in non-professional athletes is correlated with increasing duration of training (28). Li concluded that sampling time is considered a major factor for measurement and assessment of all cardiac biochemical markers (29). Some experts have reported that given the nature of the enzyme release into the blood, samples should be taken by cascade method and at different times (30). However, some researchers have attempted taking samples only once after the test (6, 18, 23). Hence, the difference in measurement time in different studies shows different troponin values. Moreover, training intensity is one of the strongest predictors of increased cTn levels. More intense training may exert pressure on the heart, resulting in release of cTn (14). An increase in the heart load can temporarily accelerate apoptosis and myocardial reconstruction. A negligible heart damage and its subsequent reconstruction is part of the natural process of myocardium, which temporarily increases serum cTn levels after training. This increase can be attributed to renal dysfunction in removing troponin from the blood circulation. Because blood flow significantly decreases in the abdomen and the kidneys during training, it results in reduced excretion capacity of the kidneys, which probably slightly increases serum cTn concentration (31). The present study results also showed significant increases in serum CK-MB values immediately, 1 hour and 24 hours after training compared to before training. But this increase did not exceed the limit showing acute myocardial infraction (< 24 µ/L). The values of serum CK-MB returned to the resting values 48 hours after resistance training. These results agree with those obtained by Shave et al., Karimajee et al., Ashmaig et al., and Toft et al. (22, 23, 32, 33), but disagree with the results obtained by van der Linden et al., Dawson et al., and Braunwald et al., who reported no increase in CK-MB values (6, 26, 30).
Toft et al. argued that an increase in CK-MB levels following intense training may indicate mechanical damage in the musculoskeletal system, resulting in inflammatory responses (33), with greater damage to muscle cells with higher intensity training (34). The increase in CK-MB levels in skeletal muscle may be due to an increase in satellite cells repairing musculoskeletal damage (34). The increase in CK-MB levels appears to be due to resistance training, and musculoskeletal damage caused by intense training has no effect on persistent cardiac damage, and may be due to a cytosolic leakage caused by the pressure of physiological activity (35). However, the increase in CK-MB, especially during exercise and recovery stages, reflects permeation of proteins and other substances through the muscle membrane. In addition, the increase in fluctuation of these enzymes is associated with such factors as age, gender, physical fitness, season, and training (36). Another reason for the increase in CK-MB after training could be participants’ lack of muscle fitness. All participants in the present study had no history of regular training, which agrees with the results obtained by Tobais et al., Lgor et al., and Dawson et al. indicating an increase in CK-MB due to the lack of fitness (37-39).
5.1. Conclusions
Given the prevalence of heart attacks and sudden deaths in sports seen in recent years, investigating the effects of various trainings on cTn and CK-MB is hugely important for understanding the causes of training-induced heart damage. The present study results showed a transient and reversible increase in cardiac troponin and creatine iso-enzyme. Thus, it can be stated that one session of resistance training at 70% of 1RM does not lead to a permanent increase in troponin and cardiac dysfunction. Yet, future studies should investigate the exact mechanisms of cardiac troponin release.