1. Background
Artificial antimicrobial compounds have long been used to manage and control deterioration or contamination of foods. Today, concerns and negative reactions of consumers to artificial and commercial preservatives have increased the tendency to use more natural compounds such as essences. In fact, given a serious need for replacing chemical preservatives with healthy and effective compounds, efforts have been focused on the potential applications of herbal essences. Medicinal plants, essences and their constituents present antimicrobial, antifungal and preservative properties against a wide range of microbial pathogens, including bacteria, yeasts and molds (1, 2). The extract of many plants such as Ziziphora, rosemary, thyme, lavender, mint, cumin, caraway, marjoram, pennyroyal, hyssop and ajwain contain compounds such as phenylpropanoid glycosides, polyacetylene, flavonoids, ditropan, polyphenols and flavone glucoside, which are involved in antimicrobial and antioxidant activities and widely used in traditional medicine (3-5).
With a scientific name "Hyssopus officinalis", this plant is commonly known as hyssop and belongs to the Lamiaceae family, and wildly grows in southern Europe, Central Asia, Russia and Iran. Hyssop has thick ramified roots and several 20 - 60 cm high wooden stems. This plant has small, paired, pointed and very fragrant leaves and purplish dark-blue, white and occasionally red flowers (6), and is widely used in pharmaceutical, hygiene and food industries. Hyssop has long been used to treat rheumatic pains, bruising, wounds, blood pressure fluctuations and neurological disorders such as depression and anxiety (7, 8). The aerial shoots of this perennial herbaceous plant are useful for the treatment of respiratory diseases, including asthma, bronchitis and coughs, as they contain chemicals such as terpenes, flavonoids, volatile oils, tannins and resin. The amount of essential oil is 0.3% - 1% in the vegetative body of hyssop and reportedly 0.1% - 1.8% in literature. Hyssop essential oil is bitter, spicy and dry, and its main constituents include pinocamphone, α and β-pinene, camphene and sesquiterpene alcohol. This plant’s vegetative body contains flavonoid, tannin (5% - 8%), bitter substances (3% - 6%) and other substances such as diosmin, hyssopin and mucilaginous compounds (9, 10).
Research confirms the antimicrobial and antifungal effects of hyssop, and shows that in addition to hyssop essence, its extract prevents the oxidation of fat and decomposition of pigments caused by cooking and preservation, and it can be used as a useful additive for meat processing and prevention of its oxidation and discoloration (11). The ethanol extract of hyssop has been recently reported to present protective properties for the gastric tract based on the adhesive stomach mucosal compounds, and also significant anticoagulant and antioxidant properties through reducing the production of free radicals (12). Research suggests that hyssop essence has special antimicrobial effects on bacteria such as Streptococcus pyogenes, Candida albicans, E. coli, and Staphylococcus aureus (13). In 2012, Marino et al. studied the effect of the main compounds of hyssop essence on six gram-positive and nine gram-negative bacteria, and reported that phenolic compounds and their most important ones carvacrol and thymol are very effective herbal compounds in microorganisms (14). Researchers associated the proper and effective dose of hyssop essence to several factors, including the consumer’s age and health status. Further scientific information is, however, needed for determining the proper dose range for hyssop essential oil. Medicinal essences and plants provide a wide range of antimicrobial and antioxidant properties. Using these compounds is also very effective as they lack the side-effects of chemicals. Identifying and investigating the amount of effective compounds contained in native essences and medicinal herbs is therefore crucial. Given that this amount differs with climate and in different parts of the plant, several experiments are required to determine the amount of these compounds in different regions.
2. Objectives
The present study was therefore conducted to analyze and determine the chemical compounds of the ethanol extracts of Hyssopus officinalis collected from the mountainous regions of Kurdistan, and to assess their antioxidant and antimicrobial properties and therefore provide a better description for these natural products and make optimal use of them.
3. Methods
3.1. Collecting the Plant and Extracting the Essence
After being collected from the mountainous regions of Kurdistan and identified and confirmed, hyssop was used in experiments to extract the plant essential oil. To extract essence, the plant was dried and milled. The volatile essential oil of the plant was then extracted using a Clevenger apparatus and through aqueous distillation. The essence obtained was dried and dehydrated using sodium sulfate, and placed in a dark glass container away from sunlight at 4 °C after passing through a 0.45-µm filter.
3.2. Bacterial Strains
Standard strains were used to evaluate the antibacterial effects of hyssop essence. These strains were collected from the Bacteriology Laboratory of Faculty of Veterinary Medicine, Tabriz University, Iran, and included E. coli (25922 ATCC) and Listeria monocytogenes (19117 ATCC). These strains were then identified using culture media and biochemical tests.
3.3. Essence Decomposition
The essence sample was decomposed using a gas chromatography device (GC-MS, model Agilent6890) equipped with a capillary column of a 30 m length, 0.25 mm internal diameter and 0.25 µm internal layer thickness. The column temperature schedule included: 2 minutes at 70°C, increasing to 220°C at a rate of 15°C /min followed by increasing and holding the temperature at 300°C for two minutes. The essence constituents were identified by comparing their mass spectrum with the bank spectrum and comparing their inhibition coefficients with reference and standard values. The relative values of the constituents were calculated by the machine software based on the total area of the peaks (15).
3.4. Measuring the Antioxidant Effect
The ability of extracts and essences tested to combine with hydrogen or electronize was measured based on the discoloration of a purple methanol DPPH solution. The stable radical DPPH was used as the reactive agent in this spectrophotometric evaluation. Fifty µL of different concentrations of the essence was added to 5 mL of a 0.004% methanol solution of DPPH and kept at room temperature for 30 minutes. Optical absorption was then read at a wavelength of 517 nm compared to the sham value. The inhibition of DPPH free radical was calculated in I% as follows:
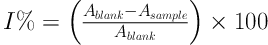
Where, Ablank is the absorption of the sham solution (containing all the reactive agents except for the essence) and Asample is the absorption of the solution containing different concentrations of the essence. The synthetic BHT antioxidant was also used as a positive control, and the tests were repeated three times, and their mean was announced as the desired value. The essence antioxidant activity is expressed as IC50, which indicates an essence concentration that causes a 50% inhibition in radical capacity (16).
3.5. Determining the Amount of Total Phenol
Phenolic compounds are measured based on Folin reagent reduction by phenolic compounds in an alkaline medium producing a blue complex showing a maximum absorption at a wavelength of 760 nm. The total amount of phenolic compounds was measured using the Folin-Ciocalteu method. Different concentrations of the essence were first prepared and 0.1 mL of each concentration was added to an Erlenmeyer flask, and 46 mL of distilled water was mixed with 1 mL of Folin-Ciocalteu reagent. Three mL of a 2% sodium carbonate solution was added to the obtained solution after 3 minutes. The solution was then placed on a moderately shaking disc at room temperature for two hours, and the absorption was read against the sham value at a wavelength of 760 nm using a spectrophotometer. Gallic acid (0 - 1000 µg per 0.1 mL) was used to plot the standard curve. The total amount of phenolic compounds contained in the essence was calculated using the equation derived from the standard curve, and the results were expressed in micrograms of gallic acid per milliliters of the essence (17).
3.6. Antimicrobial Activity Measurement
MIC and MBC were measured using microdilution. One hundred µL of the liquid medium and 100 µL of the prepared bacterial suspension (with a bacterial concentration of 1.5 × 106 per mL) were added to each well. One hundred µL of the essence or extract was then added to the first well in each row. After mixing its content, 100 µL was removed from the first well and added to the next well. A control row was considered for each test row associated with an essence or extract, but no bacteria were added to the control row. After 24 hours of incubation at the right temperature, the results were assessed in terms of opacity. The last well with no observed growth was considered the MIC. To determine MBC, 10µL of the wells’ content were cultured on Mueller Hinton agar (Merck-Germany) after 18 hours of incubation. A petri dish was incubated for 18 hours to investigate the bacterial growth. The minimum concentration of the essence or extract in which 99.9% of the bacteria presented no growth was ultimately reported as MBC. All the tests were repeated three times (18, 19).
4. Results
Table 1 present the analysis results of the essence using the GC device. Fourteen compounds comprising 71.45% of the total constituents were identified in the essence of Hyssopus officinalis. The major components of this essence were camphor (23.61%) and β-pinene (21.91%). Other important constituents included 1,8-cineole (14.25%), α-pinene (4.09%) and bicyclo hept-2-en-2-meta. The proportion of other compounds was below 2%.
| Peak N° | Component | Peak Area, % |
|---|---|---|
| 1 | Camphor | 23.61 |
| 2 | 1,8-Cineole | 14.25 |
| 3 | β-pinene | 21.91 |
| 4 | α-pinene | 4.09 |
| 5 | β-caryophyllene | 0.98 |
| 6 | Bicycloheptane | 1.8 |
| 7 | Bicyclohepta.2-n | 2.98 |
| 8 | Caryophyllene | 0.93 |
| 9 | Camphene | 0.34 |
| 10 | β-elemene | 0.24 |
| 11 | α-humulene | 0.16 |
| 12 | Hexananl | 0.05 |
| 13 | Pentadecane | 0.08 |
| 14 | Naphthalene | 0.03 |
| Total | 71.45 |
Table 2 present the results of evaluating the inhibitory strength of free radicals and the total phenolic content of the essence. The results show that, 50IC equals 11.23 µg/mL for this essence, which is weaker compared to butylated hydroxytoluene (0.7 µg/mL).
| Sample | Antioxidant Activity (IC50 *, µg mL-1) | Total Phenolic Content (mg GAE/L) |
|---|---|---|
| Hyssopus essential oil | 11.23 ± 0.02 | 23.16 ± 0.05 |
| BHT | 3.01 ± 0. 7 |
The total phenolic content of the essential oil reached 23.16 mg of gallic acid per gram of the essence.
The results obtained suggest an MIC of 156.25 µg/mL and an MBC of 312.5 µg/mL in the study bacteria (Table 3).
| Bacteria | MIC, mg/mL | MBC |
|---|---|---|
| E. coli O157; H7 | 156.25 | 312.5 |
| L. monocytogenes | 312.5 | 625 |
Abbreviations: MBC, minimal bactericidal concentration; MIC, minimum inhibition concentration
5. Discussion
The chemical composition of hyssop essential oil has been studied in literature. Fathiazad et al. reported myrtenyl acetate (74.08%), camphor (76.6%), germacrene (3.39%), spathulenol (2.10%), caryophyllene oxide (2.13%) and β-caryophyllene (2.1%) to be the main constituents of hyssop essential oil (20). A study conducted by Salma in Egypt found β-pinene (19.6%), pinocamphone (19.2%) and camphor (3.16%) to be the major constituents of the essence of this plant (21). Isopinocamphone (57.27%), β-pinene (7.23%), terpinen-4-ol (7.13%), pinocarvone (6.49%), carvacrol (3.02%), p-cymene (2.81%) and myrtenal (2.32%) were also found to be the main chemical components of the essence of hyssop collected from the southeastern region of Anatolia, Turkey (22). Research suggests that several factors affect the composition of the essences extracted from a special plant species, including the region geographical conditions, harvesting season, plant's age, agricultural operations, plant growth stage and extraction methods and conditions (23).
DPPH free radical scavenging is considered a simple and very fast method for determining antioxidant activities. The antioxidant effect on inhibiting the DPPH radical is associated to the ability of releasing a hydrogen atom or eliminating free radicals. The inhibitory activity of free radicals depends on the concentration of essences and extracts, which present better protective effects with lower levels of 50IC. The antioxidant properties of essential oils can be generally attributed to the phenolic composition, especially camphor and β-pinene. In a study conducted by Fathiazad et al. in 2011, 50IC was reported to be 25 µg/mL in hyssop (20), which shows a lower antioxidant strength compared to the present study hyssop, and can be explained by environmental and climate conditions. Hosseini et al. investigated the effects of the antioxidant strength of certain herbal essences, and showed that Zataria multiflora Boiss presents the highest inhibitory level with an inhibitory strength of 667 µg/mL, which is lower than the antioxidant strength of hyssop reported in the present study (24).
The antioxidant activity of essential oils depends on their structural properties and other factors, including concentration, temperature, lighting, type of substrate and the physical status of the system (25). Medicinal plants are rich sources of natural antioxidants, which can generally eliminate free radicals and superoxide and hydroxyl radicals by transferring free electrons. Phenolic compounds have a high antioxidant power and constitute the secondary metabolites of many plants.
Ahmadi et al. examined the hyssop extract in terms of its phenolic content, and observed an average of 3.113 mL of gallic acid-based phenolic compounds per gram of this extract (26). Soleimani et al. reported the total phenolic content of hyssop extract to be 200 mg based on gallic acid (27).
Hamedi et al. conducted an In vitro and cheese model study of anti-listeria effects of the essences of mentha pulegium (pennyroyal), tarragon and mint alone and with monolaurin, and found the mint essence with an MIC of 0.2% to present the highest anti-listeria effect. The combination of monolaurin with each of these essences increased the anti-listeria effect, and MIC was found to be 0.66% in tarragon plus monolaurin. These results showed that using low concentrations of these substances in combination can have positive antimicrobial effects on foods (28), which is consistent with the present study.
Mendoza-Yepes et al. investigated the antimicrobial effects of a mixture of herbal essences (DMC) containing 50% rosemary, Salvia officinalis and Citrics plus 50% glycerol on Listeria monocytogenes and E. coli O157:H7 in Spanish soft cheese. According to the obtained results, DMC exerts bacteriostatic effects on Listeria monocytogenes at a concentration of 2500 ppm, although it is ineffective in controlling E. coli growth (29). In contrast, the present research revealed that the essence used exerts antimicrobial effects on both of the bacteria.
In line with the present study, the simultaneous effect of pennyroyal and mint on E. coli was investigated, and pennyroyal MIC was found to be 5000 µg/mL on bacillus cereus and 4166 µg/mL on E. coli, and the mint essence was 100 mg/mL on both bacteria. Investigating the effects of three species of Satureja on Salmonella paratyphi, Sefidkon et al. found the Satureja essence to present inhibitory properties at concentrations of 2.5% and 5%, which presents lower antimicrobial properties compared to the hyssop essence (30).
The differences observed between different studies concerning the antimicrobial and antioxidant properties of different essences can be explained by the differences in the chemical compositions of plants, in methods and in their kinetics, which are affected by genetic factors, climate, environment and harvesting season. The amount of phenolic compounds was generally found to be positively associated with antioxidant activities.
5.1. Conclusion
Given the obtained results and the need for using natural preservatives, hyssop essential oil is recommended to be optimally used in the food industry to increase the shelf-life of food products and protect them against oxidizing and microbial factors and to control foodborne microbial diseases. Future studies are recommended to focus on this essence as a component of food models and in combination with other essences.
