1. Background
Brucellosis is the most common zoonotic disease in developing countries transmitted through direct or indirect exposure to infected livestock (1). Although food hygiene has improved, brucellosis continues to exist in many parts of the world, especially in developing countries (2, 3). The disease is endemic in the Middle East (including Iran), India, Mexico, South and Central America (4). Brucellosis is found in all provinces of Iran, but recently, the highest incidence rate was reported in Azarbaijan, Hamedan, Lorestan, Markazi and Kermanshah provinces (5).
In industrialized countries, brucellosis is the most common occupational disease among middle-aged men exposed to livestock or men using unpasteurized animal products (6). Due to the widespread clinical manifestations of brucellosis, the diagnosis is based on clinical findings, epidemiological features, and serum tests (1). According to data from the Ministry of Health between 2010 and 2014, about 12248 and 20117 cases of brucellosis were reported, which indicates an increase of 70% (7). The reason for the increased incidence was the lack of funding for vaccine production at Razi Institute and purchase of the vaccine from abroad. Once necessary funds were allocated for vaccine production, vaccine import, vaccination was resumed for brucellosis in 2015, and human brucellosis incidence decreased in 2016 (8).
Brucellosis is a common zoonotic disease with severe consequences for humans. The disease has a wide range of clinical sings and can lead to severe complications in musculoskeletal, digestive, genital, blood, cardiovascular, respiratory and central nervous systems. Control of brucellosis in humans depends on reducing the rate of infection in livestock and eradicating the infection in infected livestock through general animal vaccination and care (9-11).
Brucellosis is a major problem in several parts of the world and in some regions of Iran, including Kermanshah province, where livestock is a source of employment and income for many people (12, 13).
Investigating and controlling brucellosis in a region or country require improvement of policies and decisions, also access to accurate epidemiological and clinical information (14).
Based on studies, diagnosis may be delayed due to the lack of a clinically relevant factor in patient’s condition. This may sometimes cause a delay in diagnosis by the physician. In this situation, it is better to use laboratory diagnostic methods to confirm the disease (15).
Lack of knowledge about brucellosis is one of the main factors in controlling the disease, especially among rural and nomadic populations of Kermanshah and Iran. Farmers who do not have enough information about the disease are most affected by pathogens and the number of patients in rural areas is significantly higher than that of in urban areas (16).
Lack of personal protective gear and unprotected contact with animals as well as use of unpasteurized dairy products are the most important causes of the incidence of this disease in Iran, including in Kermanshah province (17). This study was conducted due to the increasing incidence of brucellosis, especially among people who are in contact with livestock and consume unpasteurized dairy products as well as residents of rural areas of Kermanshah province.
2. Objectives
The aim of this study was to evaluate the epidemiology and clinical status of patients with brucellosis recorded in Kermanshah Health Center.
Due to the increased incidence and also clinical complications of brucellosis in recent years, this study was performed to evaluate the epidemiology and clinical analysis of brucellosis in a 5-year period from 2012 to 2016. This study is based on a questionnaire containing demographic information (age at diagnosis, gender, occupation, ...), epidemiologic, clinical and paraclinical features including history of contact with livestock, history of suspicious dairy consumption, family history of disease, and history of livestock vaccination, diagnostic measures (time of onset and presentation of clinical symptoms, type of symptoms, time of diagnosis). We evaluated the results of patients’ diagnostic tests according to different criteria from their records.
3. Methods
In this study, all patients with brucellosis whose data were recorded over a five-year period at Kermanshah Health Center were enrolled. The data included clinical signs and laboratory results (Wright test at least 1:80, and ME 2 at least 1:40) indicating brucellosis according to ‘national guidelines for brucellosis’. Demographic information (age at diagnosis, gender, occupation, ...), epidemiologic, clinical and paraclinical characteristics including exposure to livestock, history of suspicious dairy consumption, family history of diseases, and history of livestock vaccination, diagnostic measures (time of onset and presentation of clinical symptoms, type of symptoms, time of diagnosis), and the results of the diagnostic tests of the patients were evaluated according to different titer and kind of medications used after the diagnosis within the specific period of time.
3.1. Ethical Considerations
All ethical principles including avoidance of potential harm to participants, confidentiality of information obtained at the beginning or during the study, the three principles of respect for the individual, beneficence and justice, as well as ethics in writing and publication of the findings were observed.
3.2. Statistical Analysis
In order to describe participant’s information, descriptive analysis techniques such as mean and standard deviation for quantitative variables and frequency percentage for qualitative variables were used. Chi-square test was used to analyze the qualitative variables. Independent t-test was used to compare a quantitative variable and a binary qualitative variable. SPSS V. 21 software was used for data analysis. Totally, chi-square and t-test were used for data analysis. In all tests, P < 0.05 was considered significant.
4. Results
The total number of people with brucellosis was 2714, of whom 1502 (55.3%) and 1212 (44.7%) were male and female, respectively. From all the participants, 2345 (86.4%) were rural residents, 322 (11.9%) were urban residents and 47 (1.7%) were nomads. The mean age of the patients was 35.05 ± 17.35 years and the most common age group was 21 - 30 with a frequency of 21% (Table 1).
| Variable | Urban | Rural | Nomad |
|---|---|---|---|
| Male, % | 6.54 | 47.75 | 1.05 |
| Female, % | 5.36 | 38.65 | 0.65 |
Residence of Patients with Brucellosis in Kermanshah Province (2012 - 2016)
The most common clinical signs and findings were musculoskeletal pain and back pain in 89.8%, fever in 81.8%, weakness and anorexia in 69.5%, weight loss in 46.6%, adenopathy in 22%, splenomegaly in 6.7%, megalohepaty in 5.7% and depression in 34%. Tables 2 and 3.
| Variables | Having Livestock | Without Livestock | Vaccination of Livestock | No Vaccination of Livestock |
|---|---|---|---|---|
| Individual with brucellosis, % | 83.90 | 16.10 | 60.90 | 39.10 |
Status of Owning Livestock and Their Vaccination in People with Brucellosis in Kermanshah Province (2012 - 2016)
| Variables | Rancher | Rancher-Farmer | House Keeper | Student | Worker | Child | Others | House Keeper-Rancher | Farmer | Butcher |
|---|---|---|---|---|---|---|---|---|---|---|
| Male, % | 16.65 | 4.75 | 0 | 8.40 | 1.81 | 1.95 | 7.70 | 0 | 13.22 | 2.50 |
| Female, % | 2.50 | 0.43 | 30.39 | 3.38 | 0 | 1.14 | 0.88 | 5.23 | 1.36 | 0 |
Occupational Status of Patients with Brucellosis in Kermanshah Province (2012 - 2016)
There was a statistically significant difference between years of incidence and livestock vaccination history (P < 0.001).
The results of Wright test are as follows: 1/80 in 19.2% of patients, 1/160 in 33.1%, 1/320 in 26.6%, 1/640 in 14% and 1/1280 in 7.2%. The results of 2ME test are as follows: 1/40 in 21.6% of patients, 1/80 in 36.8%, 1/60 in 25.4%, 1/320 in 10.6%, 1/640 in 5.2% and 1/1280 in 0.3%.
Given that multidrug treatment is used for brucellosis, 12% of patients used rifampicin and doxycycline, 6.22% used rifampin and gentamicin, 12.01% used rifampin and streptomycin, 12.97% used rifampicin and co-trimoxazole, and 11.80% received rifampin and tetracycline. Furthermore, 3.5% of patients did not use any medication, 75.2% took two medications, 20.4% used three medications and 0.9% used four medications.
Regarding the duration of treatment, 22.6% of patients were treated for less than 56 days, 3% 15 - 25 days, 72.8% 29 - 56 days, 0.1% 15 - 21 days, 0.3% 8 - 14 days, 0.6% less than two weeks, 0.3% 7 - 8 weeks, 0.2% 5 - 6 weeks and 0.1% more than two months.
The highest incidence rate of brucellosis was observed in Dalahoo as 59.18% (Table 4).
| Town | West Eslam abad | Paveh | Salas babjani | Javanrud | Dalahoo | Ravansar | Songhor | Sahne | Ghasre shirin | Kermanshah | Kangavar | Gilangharb | Harsin | Sarpol zahab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population of town | 140876 | 60431 | 35219 | 75169 | 35987 | 47657 | 81661 | 70757 | 23929 | 1083833 | 76216 | 57007 | 78350 | 85342 |
| Incidence rate | 25.52 | 3.9 | 10.81 | 19.86 | 59.18 | 16.91 | 29.36 | 26.61 | 20.88 | 14.46 | 30.56 | 52.43 | 52.37 | 30.14 |
Incidence Rate of Brucellosis (per 100000) for each Town in Kermanshah Province (2012 - 2016)
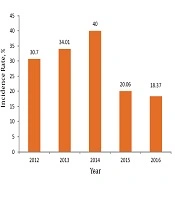
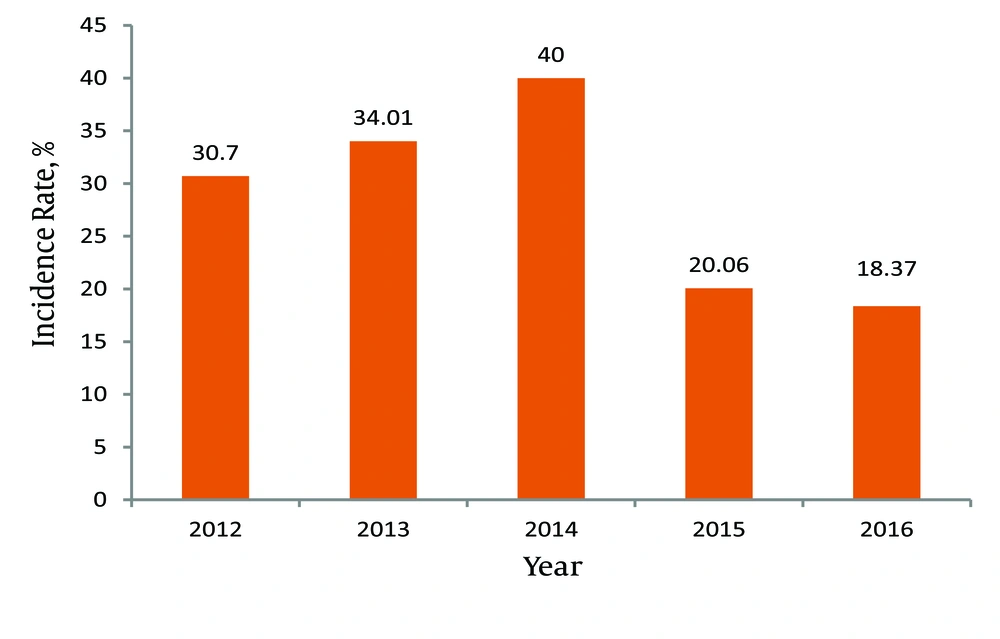
The incidence and prevalence rates of brucellosis in Kermanshah province increased from 2012 to 2014, and declined since the implementation of the SHEEP project in subsequent years (2015 and 2016) (Table 5, Figure 1).
| Variablea | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|
| Incidence rate, % | 30.70 | 34.01 | 40 | 20.06 | 18.37 |
Annual Incidence Rate of Brucellosis in Kermanshah Province (2012 - 2016)
5. Discussion
The incidence rate of brucellosis in Kermanshah province was on average 28.64 per 100000 people during 2012 - 2016. According to the Ministry of Health classification of provinces of the country for the incidence of brucellosis, with this incidence rate, Kermanshah is one of the areas with moderate infection rate (incidence rate of 21 - 40). We found the incidence rate of 28.64 per 100000 people in this study, which is considered a moderate infection rate.
In the cross-sectional study of Arghand et al. (2010 - 2014) which is conducted in Kermanshah, the prevalence of neurobrucellosis in 24 patients with brucellosis was 8.3%. Of these patients, 41.7% were male and 58.3% were female, with a mean age of 40.71 ± 14.3 years (range: 20 - 68 years), 54.2% had a history of unpasteurized dairy consumption, 62.6% lived in rural areas and 20.8% had livestock-related jobs. The most specific signs were observed in patients with neurobrucellosis are psychologic signs (25%), reduced consciousness level (20.8%), stiffness (20.8%), radiculopathy (16.7%), and seizures (3.8%), in decreasing order. One case had neuritis signs. In a cross-sectional study by Sayyad et al. (18) (2010 - 2014) in Kermanshah province conducted on 475 patients, just 289 patients met the inclusion criteria. The most common clinical findings were fever (0.83%), splenomegaly (34.6%), spine tenderness (12.8%) and hepatomegaly (8.6%). Of 289 participants, 96 patients (33.2%) had liver and biliary tract involvement. Of these, 52.1% were male and 47.9% were female, with a mean age of 44.1 ± 18.4 years. Among patients with hepatobiliary involvement, the most common clinical findings were weakness and lethargy (0.91%), fever (87.3%), hepatomegaly (34.7%) and splenomegaly (30%). Hematologic abnormalities were seen in 51.6%, leukopenia in 20% and thrombocytopenia in 27.4%.
A cross-sectional study by Mehdizad and Khademi (19) on 4398 patients with brucellosis in Kermanshah province (2010 - 2014) reported 4398 new cases in Kermanshah. The disease was slightly higher in males (55% males) than that in females. The incidence rate of the disease had an increasing trend from 2010 to 2014, which was 36.8%, 39.5%, 38.8%, 53.7% and 63.2%, respectively. This increase was further surged in the rural population, which was 100, 112, 112, 141 and 205 per 100000, respectively. Most of the patients were housewives, followed by farmer-rancher. Also, on average, 87% of patients were rural residents, and 87% had a history of exposure to livestock, with 40% reporting that they had not been vaccinated. Vaccination coverage in livestock (60%) reveals history of contact with livestock in patients (87%) (19).
In a cross-sectional study of 777 patients with brucellosis in Kermanshah province (2011), the minimum incidence rate of the disease was 39.9 per 100000. Dalahoo had the highest (2215 per 100000) and Javanrood had the lowest incidence rate (12.6 per 100000). In this study, 47.4% of patients were female and about half of cases were under 30 years of age. The majority of the patients (81.9%) expressed the use of raw milk as a cause of the disease. Also 87.6% of patients were rural residents and the peak of illness was seen in spring and summer (20).
Countries with the highest incidence of human brucellosis in the Middle East include Saudi Arabia, Iran, Palestine, Syria, Jordan and Oman (21). Of the 2714 patients studied, 55.3% were male, which is similar to most studies in the country (22, 23) as well as other countries (Turkey (24), Saudi (25)). Contrary to the present study, in some studies conducted in Iran and in other countries, more than half of those infected are women (10, 13, 26). The mean age of the patients was 35.05 ± 17.35 years, similar to most national and international studies (13, 23, 27) (unlike one national study and one international study with a higher mean (8, 10) and some other studies in and out of the country with a lower mean (9, 24, 25). Brucellosis was more prevalent between the ages of 31 and 40 years. In one study conducted in Iran, the most reported cases were in the age group of 15 to 44 years (28), 15 to 20 years in another (29), 10 to 19 years in another (30), and 40 to 49 in another one (31). We observed brucellosis, similar to other studies, more prevalent among housewives, ranchers, and farmers (22, 29). Furthermore, it was more common in summer in Kermanshah province (32.20%) than in other seasons. Although it is prevalent in all seasons, it is more common in spring and summer, the season of breeding and lactation. Similar results have been noted in most articles in and out of the country. One study out of Iran showed the disease was most prevalent in summer, autumn, and winter (7, 13, 23, 29, 31-33).
According to two reports from other countries, climate may have affect breeding and lactation. Climate changes due to the environmental and global warming, have had many effects on mammal’s health, production and reproduction, which has the consequences as slow growth, reduced reproduction, increased potential risk for disease, and ultimately delayed onset of lactation (34, 35).
The most common route of transmission is consumption of suspicious dairy products and simultaneously contact with livestock, which is in agreement with most studies in and out of the country (13, 17, 23, 36-38). In terms of clinical signs, most studies in and out of the country have reported similar findings including fever, anorexia, weight loss, low back pain, and musculoskeletal pain (13, 25, 39). Some studies in and out of the country have also found other signs such as anemia and night sweats, headaches, hepatomegaly and splenomegaly (12, 39). In the present study, the majority of patients (33%) had a titer of 1/160 in Wright test, and 1/80 in 2ME test. In studies out of Iran, Wright test was positive in 33 cases (41%). In a study in Fars province in Iran, 2ME test was positive in 30 cases (5.5%) (10, 26). In the present study, the majority of patients were from rural and nomadic areas. Findings from other studies in many cases confirm this result, however, one study in Iran reported the majority of patients live in the city (23, 26). The family history of patients in this study was 18.5% (34), which is almost in agreement with some other studies with 15% and 13%, and also consistent with another study (31) with 20%. Meanwhile, it was more in other studies even more than 40%. These indicate infection transmission through animals rather than human-to-human transmission (38). However, in a study in Turkey, genetics have been evaluated as an environmental factor in potential risk for brucellosis in children (12, 33, 36, 40). Studies show that delayed diagnosis may be an important clinical feature affecting patients with brucellosis, which indicates that both patients and physicians may have a significant delay in diagnosis. It has been suggested that diagnostic procedures need to be improved prior to the onset of symptoms. Also, physicians should be aware of the source of the infection and disease because it is difficult to diagnose during mild stages. Despite difficult diagnosis, screening for at-risk people and pregnant women can be helpful in detecting the disease (34, 41). Lack of awareness about the disease is one of the main reasons for failure to control the disease, particularly among the children and teenagers of nomads and rural areas, who are more likely to be exposed to livestock and brucellosis. They still do not have sufficient basic information about the disease. Various studies in the country indicate that the general public has little information about the disease (12, 42).
5.1. Conclusions
Due to the prevalence of the disease in the rural areas of Kermanshah province, timely and early detection of the disease is necessary for residents in these areas. In addition to vaccinating livestock, it can also be helpful to educate the community about the impact of contaminated livestock, refraining from unpasteurized dairy products, and production and consumption of healthy dairy products.