1. Background
In recent years, vitamin D (calciferol) has attracted a lot of attention in field of children’s healthcare. The presence of vitamin D receptors on various cells has extended its effects in the body. Vitamin D can play an important role in bone mineralization by increasing absorption of calcium from the small intestine, balancing the function of osteoblasts and osteoclasts and increasing kidney reabsorption of calcium. It is also involved in reducing inflammatory cytokines and increasing anti-infective molecules (1-6).
Sunlight is known as a major source of vitamin D by providing nearly 90% of vitamin D in human body. It can supply our body’s required vitamin D by exposing daily to sunlight with uncovered skin of face, arms, back and legs for at least 5 to 30 minutes between 10 am and 3 pm. Currently, food sources and vitamin D supplements are very important for us due to lifestyle changes, air pollution and decreases in duration of sunlight exposure (2, 7-11).
The main problem with vitamin D deficiency is its obscure and nonspecific symptoms which fails early diagnosis. Children presenting with delays in walking and teeth growing, irritability, seizure and frequent infections are common. These problems can lead to bone diseases such as rickets, autoimmune diseases such as multiple sclerosis, chronic urticaria, cardiovascular and respiratory diseases such as cardiomyopathies and asthma and also mental disorders such as schizophrenia, depression, autism and sleep disorders (1, 2, 7-9, 12-16).
One billion people worldwide suffer from vitamin D deficiency, with a high prevalence in the Middle East countries such as Iran, especially in children under 12, with a prevalence range of 25 to 85% (1, 2, 8, 12, 13, 17-20).
Since Iran has adopted the policy to prevent vitamin D deficiency in children under one year old by administering vitamin A + D drop and that vitamin D requirement increases with growth and ossification, it appears children older than one year need to be addressed (2, 21).
To prevent the increasing prevalence of vitamin D deficiency and incidence of irreparable complications, we need to treat vitamin D deficiency in younger ages (22).
2. Objectives
The purpose of this study is to evaluate the level of vitamin D among children of 1 - 6 years old in Tehran from 2016 to 2017 and to investigate the relation between prevalence of vitamin D deficiency and variables of age, sex and body mass index (BMI).
3. Methods
3.1. Study Design
This cross-sectional analytical study was conducted among children of 1 - 6 years old presenting to pediatric clinic of Javaheri Hospital affiliated to Islamic Azad University of Medical Sciences in Tehran, Iran from 2016 to 2017.
The implementation protocol was approved by the ethics committee of Islamic Azad University of Medical Sciences (ethics code: IR.IAU.TMU.REC.1395.87). The participants entered the study after obtaining informed consent from their parents. Data and information obtained were considered confidential and used without mentioning children’s name.
3.2. Study Population
All children between one and six years old presenting to the clinic for routine examination or treatment and without having any past medical history of liver or renal diseases, malabsorption and cancer or using any medicine affecting vitamin D metabolism like anticonvulsants entered the study.
3.3. Sample Size Calculation Formula
Different studies have reported different values for the prevalence of vitamin D deficiency across the globe. Based on the study of Motlaghzadeh et al. (23), the prevalence of vitamin D deficiency was considered 81.1% in this study. The sample size was determined using a 95% confidence interval (Z = 1.96 for 95% confidence interval) and margins of error of 5% (d = 0.05). The calculated sample size was a minimum of 235 whereas 288 children were selected by convenience sampling method.
Z = 1.96; P = 81.1%; d = 5%; α = 0.00.
3.4. Anthropometric Measurements
Weight was measured using a standard scale (Seca, Hamburg, Germany) while the children were wearing light clothing and without shoes, and rounded to the nearest 0.1 kg. For children below 2 years of age Seca baby scale was used. In children younger than two years, recumbent length was measured to the nearest 1 cm using a length board placed on a flat, stable table. For children above 2 years of age standing height was measured by a measuring tape to the nearest 1 cm.
BMI was calculated by the standard method of division of weight on squared height in kg/m2, and rounded to the nearest 0.01 kg/m2. BMI Z-score was calculated using the international growth standard distribution charts of the World Health Organization (WHO) (www.who.int/childgrowth/standard). BMI over 97th%ile was classified as obese, between 85th to 97th%ile as overweight, between 15th to 85th%ile as normal, between 3rd to 15th as thin and below 3rd%ile as severely thin.
A venous blood sample (5 - 10 mL) was taken from all children at laboratory of Javaheri Hospital. All samples were centrifuged, and serum and plasma were separated and stored at -20ºC until analysis. Afterward, 25(OH) vitamin D levels were measured using radioimmunoassay (RIA) method by same ELISA kit used for all samples.
The conventional number for vitamin D levels was specified based on the level determined by WHO in 2015 as follow:
Vitamin D level ≥ 30 ng/mL: normal
Vitamin D level 20 - 29 ng/mL: mild deficiency
Vitamin D level 10 - 19 ng/mL: moderate deficiency
Vitamin D level < 10 ng/mL: severe deficiency
3.5. Statistical Analysis
All data were analyzed by SPSS software version 16. Quantitative variables were described as mean ± standard deviation and qualitative variables were described as frequency and percentage. Chi-square test was used to measure frequency of variables, and independent sample t-test was used to measure the qualitative variables. One-way ANOVA was used for intergroup comparison. Vitamin D level was a dependent variable while age, gender, height, weight and BMI were independent variables. Also, linear regression test was used to assess the relation between age and vitamin D deficiency. P value < 0.05 was considered significant for the test.
4. Results
In the present study, 288 children of 1 - 6 years old were examined among who, 49% (136 persons) were female and 51% (152 persons) were male. The mean age was 2.75 ± 1.74 years and the mean weight was 14.72 ± 5.66 kilogram. The mean height was 95.54 ± 15.43 centimeter and the mean BMI was 15.69 ± 2.13 kg/m2. The prevalence of BMI groups of severely thin, thin, normal, overweight and obese was 8.7%, 19.1%, 58.3%, 7.3% and 6.6%, respectively. Among the investigated variables, there was a significant relation between weight and gender (P value = 0.01), such that boys had higher values than girls (Table 1).
| Variable | Total | Boys | Girls | P Valueb |
|---|---|---|---|---|
| Weight, kg | 14.72 ± 5.66 | 15.47 ± 6.29 | 13.88 ± 4.75 | 0.01 |
| Height, cm | 95.54 ± 15.43 | 96.71 ± 16.22 | 94.24 ± 14.34 | 0.17 |
| Age, y | 2.75 ± 1.74 | 2.84 ± 1.79 | 2.65 ± 1.68 | 0.36 |
| BMI, kg/m² | 15.69 ± 2.13 | 16.02 ± 2.27 | 15.32 ± 1.91 | 0.005 |
| 25(OH)D, ng/mL | 37.92 ± 26.48 | 38.86 ± 26.52 | 36.86 ± 26.50 | 0.522 |
Abbreviation: 25(OH) D, 25-hydroxy vitamin D.
aValues are expressed as mean ± SD.
bP value, refers to the comparison of each variable between sexes.
Mean level of 25(OH) vitamin D among the study population was 37.92 ± 26.48 ng/mL. Fifty-one percent of the children had vitamin D deficiency (serum 25(OH) vitamin D < 30 ng/mL). Of these, 4.51% had severe deficiency (serum 25(OH) vitamin D < 10 ng/mL), 18.40% had moderate deficiency (serum 25(OH) vitamin D = 10 - 20 ng/mL) and 28.12% had mild deficiency (serum 25(OH) vitamin D = 20 - 30 ng/mL) (Table 2).
| Vitamin D status | Boys | Girls | Total |
|---|---|---|---|
| Severe deficiency, < 10 ng/mL | 7 (4.60) | 6 (4.41) | 13 (4.51) |
| Moderate deficiency, 10 - 20 ng/mL | 23 (15.13) | 30 (22.05) | 53 (18.40) |
| Mild deficiency, 20 - 30 ng/mL | 45 (29.60) | 36 (26.47) | 81 (28.12) |
| Normal, ≥ 30 ng/mL | 77 (50.65) | 64 (47.05) | 141 (48.95) |
| Total | 152 (100) | 136 (100) | 288 (100) |
aValues are expressed as No. (%).
bVitamin D status was not dependent on sex (P = 0.50).
Frequency of vitamin D deficiency was 49.35% in boys and 52.95% in girls. There was no significant relation between gender and vitamin D deficiency (P value = 0.36) (Table 3).
| Modela | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | 95% Confidence Interval for B | ||
|---|---|---|---|---|---|---|---|
| B | Std. Error | Beta | Lower Bound | Upper Bound | |||
| Constant | 44.774 | 6.630 | 6.753 | 0.000 | 31.724 | 57.824 | |
| Age | -3.479 | 0.886 | -.230 | -3.926 | 0.000 | -5.223 | -1.735 |
| Sex | 2.777 | 3.077 | 0.052 | 0.903 | 0.367 | -3.279 | 8.834 |
| BMI Range | -0.542 | 1.686 | -0.019 | -0.322 | 0.748 | -3.861 | 2.777 |
aDependent Variable: 25(OH) Vitamin D level.
The mean BMI level was 15.48 ± 2.06 kg/m2 in the children with vitamin D deficiency and 15.91 ± 2.20 kg/m2 in children with normal levels of vitamin D.
There was no significant relation between their BMI and vitamin D deficiency (P value = 0.74) (Table 4).
| Vitamin D State | Numberr | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Normal | 141 | 15.9132 | 2.20170 | 0.18542 | 15.5466 | 16.2798 | 11.98 | 26.69 |
| Deficiency | 147 | 15.4833 | 2.06003 | 0.16991 | 15.1475 | 15.8191 | 12.17 | 25.71 |
| Total | 288 | 15.6937 | 2.13771 | 0.12597 | 15.4458 | 15.9417 | 11.98 | 26.69 |
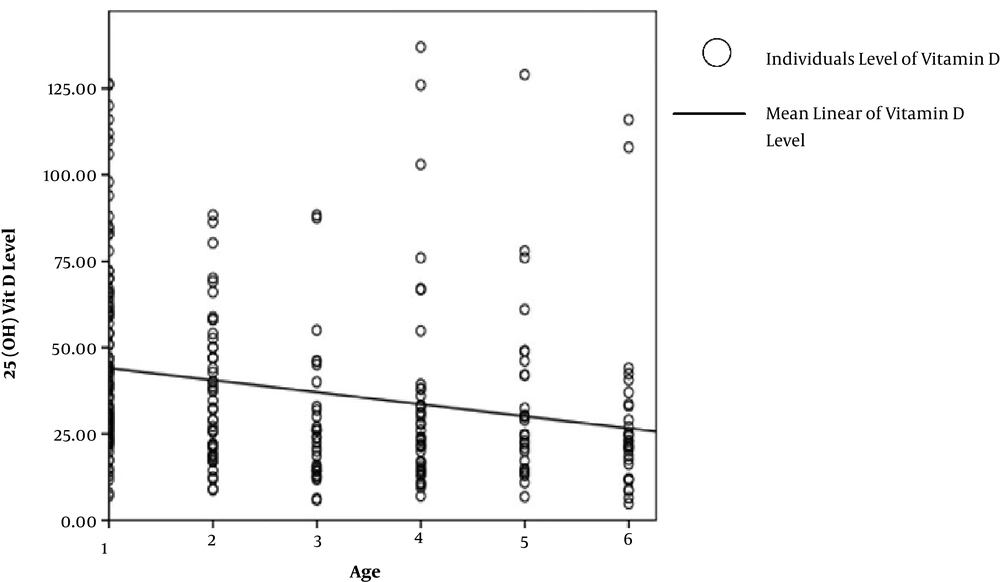
Only the children’s age had a significant relation with serum vitamin D level (P value = 0.00) and by linear regression analysis with B = -3.47 this relation had a descending order which means with every one year of age increase, serum level of 25(OH) vitamin D reduces by 3.47 ng/mL (Figure 1).
5. Discussion
In the present study, the prevalence of vitamin D deficiency was 51% in children of 1 - 6 years old in Tehran, 52.8% in boys and 47.2% in girls. Among the investigated variables, serum level of 25(OH) vitamin D had a significant relation only with age. Serum level of 25(OH) vitamin D was reduced by 3.47 ng/mL with every one year increase in age. The present research investigated especially the prevalence of vitamin D deficiency among children of 1 - 6 years old for the first time in Tehran. The reason behind choosing this age group was the changes in dietary habits and the duration of outdoor activities after the child’s entry to school, distributing vitamin supplements after six years old at schools, and breastfeeding and receiving vitamin D supplements before one year old. Due to the importance of vitamin D deficiency, especially in early childhood, many studies have investigated its prevalence and related factors. In this regard, three studies were conducted in Tehran. Mohammadian et al. (18) studied the prevalence of vitamin D deficiency among 215 children aged two and seven years old and reported deficiency (level of 25(OH) vitamin D lower than 20 nmol/L as 85.6%. Torkaman et al. (2) investigated the prevalence of vitamin D deficiency among 286 children younger and older than 2 years in which deficiency (level of 25(OH) vitamin D lower than 30 ng/mL) was reported as 76.2%.
Studies of Saki et al. (1) in southern Iran and Rajebi et al. (7) on vitamin D deficiency among female nurses of children’s medical center hospital claimed that the prevalence of vitamin D deficiency was increasing due to modern lifestyle like a decrease in tendency toward outdoor physical activities and a reduction in parental control on children’s diet in higher ages.
On the other hand, some studies showed higher prevalence rates for vitamin D deficiency in children compared with the present study; however, they have overlooked the effective health factor of the major organs in vitamin D production cycle. Sobouti et al. (24) studied 118 children with a mean age of 4.04 years old with different degrees of burns in Tehran for two years. The prevalence of vitamin D deficiency (level lower than 30 ng/mL) was reported 96.61%.
In a study in North Khorasan Province among 361 children of 7 - 18 years old, the prevalence of vitamin D insufficiency was 41.3%. Lower prevalence rates of deficiency could be attributed to lower pollution of the study region compared with Tehran (25).
Differences in the results of different studies can be attributed to differences in the studied age range, seasons and geographical regions with different levels of humidity, different methods of measurement (RIA ʋ HPLC) of serum level of 25(OH) vitamin D, different cut-off points for vitamin D deficiency and some diseases. However, all studies showed a critical need for more research and planning at national level to eliminate the high prevalence of vitamin D deficiency (8, 19, 26-29).
In the study of Park et al. (9) on 140 Korean children of 2 - 15 years old with non-specific back pains, the prevalence of vitamin D deficiency (level of 25(OH) vitamin D less than 20 nmol/L was reported 57.1%. Most of the children with vitamin D deficiency were older children. Beuzit et al. (30) studied different age groups of 316 children younger than 10 years old in Western Britain and reported the lowest prevalence rate in age group 0 - 18 months (8.8%) and the highest prevalence rate in age group 5 - 10 years (51.6%). On the other hand, in Jamali’s study (2013) on 250 girls of 11 - 17 years old from Rafsanjan, the prevalence rate of vit D deficiency (level of 25(OH) vitamin D less than 50 nmol/L was reported 59.6% with no significant relation between vitamin D deficiency and age (9, 30, 31).
Hovsepian et al. (32) studied 1111 individuals of 20 - 80 years old from Isfahan, and reported the prevalence of vitamin D deficiency 70.4%, which indicated that the prevalence rate was significantly higher in young women (32).
In our study, 58.3% of the children had a normal BMI and 6.6% were classified as obese. Also 55.8% of the children with vitamin D deficiency had a normal BMI, 30.6% had a lower BMI and 13.6% had a higher BMI than normal. There was no significant relation between BMI and the prevalence of vitamin D deficiency.
Motlaghzade et al. (23) studied 90 children of 2 - 14 years old and reported the prevalence of vitamin D deficiency (the level lower than 30 ng/mL) 95.6% in obese children and 66.7% in non-obese children.
Some studies have shown that prevalence of vitamin D deficiency increases with an increasing release of leptin from the body fat in people with higher weight and with controlling of vitamin D from kidneys and the relation between obesity and the reduction of sunlight exposure and physical activities (1, 33).
In this study, 49.3% of boys were vitamin D deficient with a mean 25(OH) vitamin D level of (26.52 ± 2.15 ng/mL) while 52.9% of girls were vitamin D deficient with a mean 25(OH) vitamin D level of (26.50 ± 2.27 ng/mL). Although girls had lower serum mean level and higher percentage of vitamin D deficiency, they were not significantly different. Similarly, in a study conducted on the children younger and older than two years old in Tehran, the prevalence rate of vitamin D deficiency was not related to gender. However, the prevalence of vitamin D deficiency in children of 5 - 18 years old in six provinces (West Azerbaijan, Semnan, Lorestan, South Khorasan, Khuzestan, Fars) showed a higher prevalence in girls. This higher prevalence can be attributed to Islamic dress code of girls older than nine years old, which causes less exposure to sunlight (33). Also, Mainos et al. attributed most of the vitamin D deficiency prevalence in girls of 9 - 13 years old in a city in Greece to an increase in indoor activities. Besides, some studies have attributed lower prevalence of vitamin D deficiency in men, despite having an equal BMI with women, to 10% - 15% less body fat content, which leads to less amount of vitamin D stored in body fat and more amount of vitamin D available in blood (2, 34, 35).
5.1. Conclusions
The present study was conducted in Tehran with a high genetic diversity and a high migrant population. Future research should focus on indigenous habitant with less genetic diversity to provide a more accurate estimate of vitamin D deficiency prevalence. Furthermore, studying different provinces will give us its prevalence in the country. To reduce the effect of body cover in girls and duration of sunlight exposure on the results, it is better to investigate a given season. To emphasize the importance of the prevalence of vitamin D deficiency and its impact on physical and mental health of children, some studies claimed the significant relation between the prevalence of vitamin D deficiency and cancers, infections, autoimmune diseases, and mental problems. Considering the urban lifestyle and absence of sufficient sunlight exposure, it is necessary to maintain the serum level of vitamin D in normal range (higher than 30 ng/mL) through taking at least 800 - 1000 UI supplements or through enriched processed foods on a daily basis. Currently, physicians and media emphasize the importance of vitamin D deficiency in the country in an attempt to raise the level of parental awareness. Therefore, it is recommended that some studies be conducted on the exact amount of enriched food consumption in Iran, the degree of body’s response to these nutrients through public invitation. Screening healthy children for estimating the amount of required vitamin D through medication in addition to sufficient consumption of the nutrients. Finally, countrywide planning for dairy and juice enrichment, and consuming biscuits containing 50000 IU cholecalciferol could also have significant impacts on increasing vitamin D level especially in children (12, 36-41). A study conducted by Mostafai et al. (42) revealed that daily consumption of yogurt fortified with 1000 IU vitamin D for three months could significantly increase serum level of 25(OH) vitamin D by 12.6 ng/dL.
It is also important to reduce the selection bias in the study population, as most of participants had come to the hospital for treatment, by selecting the study population from different centers related to healthy children such as schools and kindergartens.