1. Background
Nesfatin-1 is a hypothalamic anorexigenic peptide shown to counter appetite in different way from other anorectic peptide like ghrelin and leptin (1). Nesfatin-1 is cleaved from N-terminal of the precursor protein nucleobinding 2 (NUB2) and expressed in different tissues such as brain and adipose tissue and pancreatic β cells (2). Nesfatin-1 expression level has been reported 20-fold higher in the stomach oxyntic mucosa than in the brain (3, 4). Nesfatin-1 showed important role in energy hemostasis and metabolism. Intracereblo-ventricular (i.c.v) injection of NUCB2 decreased food intake, and long-term administration of nesfatin-1 reduced body weight in rats (5). Nesfatin-1 was thought to act through independent mechanism from leptin because it active property was not affected in Zucker rat in which leptin receptor was mutated (6). Nesfatin-1 also showed caused hyperpolarization in arcuate nucleus, which are important sources for neuropeptide-Y (NPY) secretion (4, 7). Oxytocin release in the paraventricular nucleus (PVN) was promoted by nesfatin-1 because it has been shown that nesfatin-1 injection to 3rd brain ventricle can activates the release of oxytocin in PVN (6, 8). Postprandial control of feeding mode and energy homeostasis may be in somehow controlled by feeding activated nesfatin-1 neurons in the PVN and supraoptic nucleus (SON) (3). Nestafin-1 plasma levels showed a significant correlation with weight-related abnormalities in health and diabetic conditions (9). Regulatory effects of nesfatin-1 on insulin resistance (IR) is somehow similar to changes in glucose homeostasis caused by exercise training stress (10) and accordingly, it is presumed that exercise-induced changes in the metabolism of glucose and insulin may influence nesfatin-1 release at different levels (11).
Impact of physical activity and different types of exercise on the release of hormones involved in food intake and energy homeostasis showed the beneficial role of exercise and physical activity on appetite regulation (12). Exercise training in different modes can decrease adiposity and affect whole-body fat metabolism (13) This is an important aspect of exercise, whose main consequences are enhancing energy expenditure and release/suppression of hormones like orexigenic (NPY , ghrelin) and anorexigenic peptide/protein (obestatin, leptin, and visfatin) in central and peripheral tissues (14-16). Furthermore, some investigations suggest that appetite suppression in acute exercise is greatly intensity related and more intense exercises can cause greater stimulation of anorexigenic signals (14).
2. Objectives
Changing hormone levels involved in energy regulation in response to different stimuli like physical stress attracted a lot of research attention. Acute effects of exercise on peptides involved in metabolic response and appetite control like nesfatin-1, may give us important insight about their roles, and these effects may persist during recovery hours after exercise (10, 17). Some types of training workouts like low volume resistance exercise in form of circuit training with minimum rest time showed to be more effective in postponing the manifestations of non-insulin-dependent diabetes mellitus compared to aerobic endurance exercise (18), Furthermore because of minimum rest time between sets and intervals of this type of exercise, circulating levels of some hormones that affect substrate utilization like GH, cortisol, and insulin are higher, which may elevate bloods glucose and lactate levels as well as nesfatin-1 concentrations (17). Therefore, we were interested in studying the effects of acute and delayed responses of plasma nesfatin-1 and IR to workouts like circuit resistance exercise that alters both aerobic and anaerobic metabolic systems.
3. Methods
3.1. Participants
Twelve healthy male non-smokers voluntarily participated in this study (Table 1). All participants completed a medical questionnaire to ensure that they were free of drug and medication, and had no history of hormonal disorders, diabetes or obesity. The Ethics Committee of Kermanshah University of Medical Sciences initially approved the experimental protocols. All participants were briefed on all study procedures and potential risks, and signed a written consent after having viewed and understood all aspects of the trials and measurements.
3.2. Experimental Design
After the determination of 1-RM, participants were asked to attend in two counterbalanced randomized sessions (exercise and control) that were performed with a seven-day interval starting at 08:00 a.m. The exercise session was conducted after the ten-minute warm-ups. Exercise session included the performance of three circuits that consist of seven exercises with ~14 repetitions for each one at 50% 1-RM and completion of three circuits lasted ~25 minutes. Two-minute and 30 seconds rest was allowed between circuits and exercises respectively (19). The resistance exercises order were as follow: bench press, leg extension, lat pull-down, leg curl, biceps curl, triceps pushdown, and squat. After acute circuit exercise, all participants had 1-hour of recovery and seated throughout this recovery time. In the control session, all participants rested for a similar time just equal to exercise session. To control for the potential effect of food and strenuous physical activity, all experiments took place after at least 10 hours fasting and all participants were asked not to have strenuous physical activity 48 hours before the start of the test. Caution was taken to ensure that the environmental conditions (ambient temperature, 22 - 24°C and relative humidity, 45% - 50% were identical during both sessions.
3.3. Body Composition and 1-Repetition Maximum (1RM)
Although all participants had experienced working with weights and resistance training, one familiarization session was planned. During this session, participants were habituated with the experimental protocols and laboratory environment setting. Further in this session, height was measured to the nearest 0.5 cm using a calibrated stadiometer (Seca 206, Seca Corp, Harmans, MD) and body mass and body percent were measured using the Body Composition Analyzer (VENUS-5.5, Jawon Medical, Seoul, Korea) with correction for light indoor clothing. According to the familiarization session, participants were asked to report to the weight training Gym for an additional session designed to determine 1RM for seven exercises including upper and lower body parts. During determining 1-RM all participants must use the correct technique for each exercise and the heaviest weight successfully lifted was considered as the 1-RM.
3.4. Blood Sampling and Analysis
Blood samples were collected from an antecubital vein (8 mL each time) at each sampling point time (before and instantly after the exercise, after 1-hour recovery and 24-hour following exercise cessation) following exercise session and corresponding time at control session. Immediately after blood sampling plasma was separated by centrifugation (2500 g, 15 minutes at 4°C) and frozen at -70°C for following analyses. Plasma nesfatin-1 was measured using human ELISA kit (Cusabio Biotech, Wuhan, China). The intra- and interassay coefficients of variation (CV) were both < 6% and sensitivity for this assay was 7.8 pg.mL-1. Insulin was measured by human ELISA kit (Mercodia Insulin ELISA, Mercodia AB, Syleveniusgatan, Sweden). The sensitivity of insulin kit was 1 mU/L, and the intra- and interassay CV were < 3.2% and < 4.7%, respectively. Plasma glucose was determined by an enzymatic, calorimetric method (Pars Azmoun, Tehran, Iran). IR was determined using Dill and Costill homeostasis model assessment (HOMA-IR) (20) as following formula:
3.5. Statistical Analysis
Statistical analyses were conducted by SPSS software for Windows, version 16.0 (SPSS Inc., Chicago, IL). Means ± SD were computed and normal distribution of all variables was evaluated using the Shapiro-Wilk test. A two-way ANOVA with repeated measures across two sessions (exercise and control) and four times (pre, post, 1-hour, and 24-hour recovery) was applied to explore differences in mean values. When ANOVA showed a significant difference, Bonferroni’s post hoc test was used to determine differences between times. Baseline values were compared using Paired t-test. Data are shown as mean (± SD) unless otherwise stated. Statistical significance was accepted as P < 0.05.
4. Results
Characteristics and body composition of the study participants are presented in Table 1. No significant differences were observed in resting levels of all factors in two sessions (P > 0.05). Results for 1-RM testing included: chest press (65.3 ± 2.7 kg), triceps pushdown (33.7 ± 3.5 kg), biceps curl (45.1 ± 1.9 kg), latissimus pull-down (51.0 ± 2.3 kg) for upper-body, and squat (159.4 ± 8.3 kg), leg curl (35.1 ± 1.2 kg), leg extension (49.8 ± 2.7 kg) for lower-body.
| Demographic | Mean ± SD |
|---|---|
| Age (y) | 22.1 ± 1.9 |
| Weigh (kg) | 68.9 ± 7.9 |
| Height (cm) | 176.2 ± 6.2 |
| BMI (kg/m2) | 22.2 ± 2.3 |
| WHR | 0.77 ± 0.04 |
| Fat percent (%) | 16.6 ± 5.1 |
| Sum of 1-RM of 7 movements (kg) | 359.4 ± 69.7 |
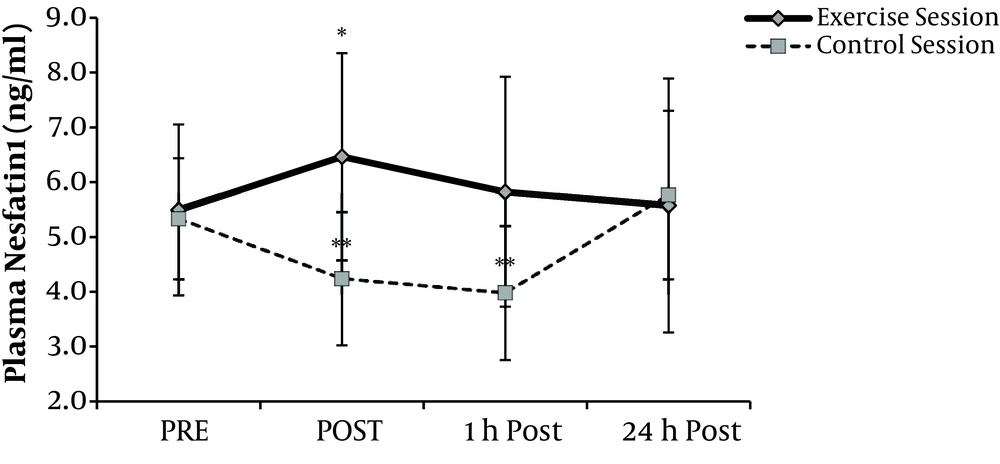
Changes in plasma nesfatin-1 levels during time series in both sessions (Figure 1), showed main effects of exercise on nesfatin-1 (P = 0.004). Post-hoc analyses revealed a significant rise at post-exercise nesfatin-1 in exercise session compared with rest values (P = 0.008). During recovery, these values decreased and reached resting levels. In the control session plasma nesfatin-1 was significantly decreased by 21% and 26% respectively at the equivalent times to post-exercise and 1h recovery at exercise sessions (P < 0.05). Compared with pre exercise values, nesfatin-1 levels remained unchanged 24-hour after at both exercise and control sessions.
Plasma Nesfatin-1 values Mean (± SD) at before (PRE), immediately after (POST), after 1-hour recovery (1h Post) and 24-hour recovery (24h Post) at both sessions. A significant main (P < 0.01) effect of exercise comparing pre levels is denoted by *. Significant (P < 0.05) difference comparing pre levels at control session denoted by **.
Statistical analyses of data comparing two sessions showed a, significant effect of acute circuit resistance exercise (Table 2) on insulin (P = 0.001) and glucose (P = 0.032) concentrations. In the exercise session, post hoc analyses revealed a significant difference between rest and post exercise values for both variables (P < 0.05). During 1-hour recovery, insulin and glucose levels returned to baseline levels. However, insulin and glucose concentrations remained unchanged compared with resting levels in 24-hour post-exercise (P > 0.05). In the control session, repeated measure ANOVA did not show any significant changes for both insulin and glucose at the time points equivalent to post exercise, 1-hour recovery and 24-hour after exercise periods (P > 0.05).
aIndicates a significant (P < 0.01) effect of exercise compared with pre-exercise levels.
bIndicates a significant (P < 0.05) difference compared with pre-exercise levels at the control session.
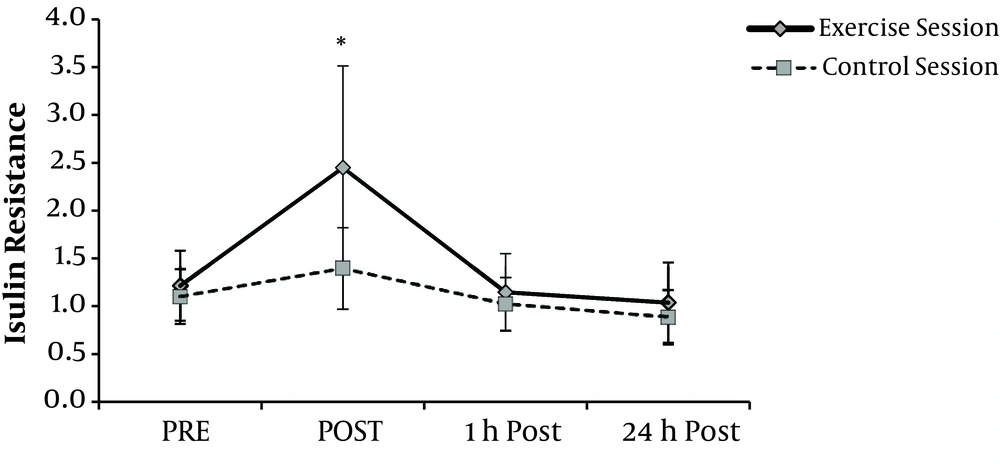
IR at exercise session showed a significantly different response compared with the control session (P = 0.013). In the exercise session, post hoc analyses showed a remarkably significant difference between rest and post exercise IR (P = 0.008). During 1-hour recovery, these values decreased and reached pre-exercise values and no changes were observed between IR values at resting and those of 24-hour post-exercise. In the control session, repeated measure ANOVA reveled that IR did not show any significant changes when comparing different times (P = 0.052) (Figure 2).
5. Discussion
The present study revealed a significant effect of acute circuit resistance exercise on plasma nesfatin-1 concentration and IR in healthy men. Our hypothesis of increased nesfatin-1 concentration immediately after exercise was supported by our findings (~19.8% increase was observed). However, during the 1-hour post-exercise, plasma nesfatin-1 returned to pre-exercise level. In contrast plasma nesfatin-1 in the control session was significantly decreased at times corresponding to post-exercise and 1-hour recovery of exercise session. This increase can be explained as that circuit resistance training session under fasting condition involved upper and lower body movements, and resulted in a physical stress that was sufficient to cause significant changes in plasma nesfatin-1 concentrations. However fasting condition in the control session that changed cellular energy status in a different way caused a significant decrease in nesfatin-1. In contrast to our study, Ghanbari-Niaki et al. reported no significant increase in plasma nesfatin-1 after interval anaerobic exercise or circuit anaerobic exercise (17). Other satiety peptides including obestatin and ghrelin showed no significant changes after circuit resistance exercise (21, 22), but Agouti-related peptide (AgRP) showed a significant increase in response to acute circuit resistance exercise (23). Mohebbi et al. revealed that intensity of exercise may have an important effect on varieties of plasma levels of nesfatin-1 after running at anaerobic threshold and maximal fat oxidation intensities (1). The plasma nesfatin-1 level is in a ranges 2 - 5 ng/mL (2.0 - 5.0 × 10 mol/L) in non-obese healthy men and eating meals or oral glucose tolerance test (OGTT) showed no effects on plasma nesfatin-1 levels in normal males (24). Nakata et al. also showed that insulin secretion in mouse islet P-cells can be enhanced with nesfain-1 through promoting Ca2+ influx by L-type Ca2+ channels (25). Because of insufficient plasma nesfatin-1 levels in normal humans/animals for influencing insulin release from P-cells, they suggested that nesfatin-1 paracrine secretion from islets themselves is responsible for increasing insulin secretion (26). Because of all body parts muscle contractions and more metabolic stress exerted on the whole body, the increase in insulin and glucose levels immediately after acute resistance exercise has a different mechanism compared with eating meals or OGTT. Based on data form Nakata et al., a significant increase in plasma nesfatin-1 in this condition may be one of the reasons for increasing insulin secretion and IR immediately after exercise. An increase nesfatin-1 after acute resistance exercise may be one of the mechanisms that can explain short-term anorexia after acute exercise which is reported by most studies (16, 27). Data from control session showed a considerable decrease in nesfatin-1 in response to fasting condition. This result is similar to that of Foo et al. that showed a significant decrease (~18%) in nesfatin-1 levels at fasting condition in rats (26). Its appears that cellular energy status affected by fasting condition causes liver ATP and glycogen depletion (hypoglycemia), which may somehow be the result of a reduction in nesfatin-1 levels, which is the best way to induce mechanisms for blockage of anorexia; however the exact mechanism responsible for these conditions is still not known well and more research is needed in this field (28). The lack of nesfatin-1 changes in 1-hour and 24-hour recovery periods in the exercise session is in agreement with Ghanbari-Niaki et al. study, probably due to the short duration of exercise protocols and low energy expenditure of this type of exercise (17), It is possible that greater total caloric expenditure by longer exercise protocols at a lower exercise intensity would have affected nesfatin-1 plasma concentrations in the recovery periods.
5.1. Conclusions
Our study demonstrated that circulating plasma nesfatin-1 was affected as a result of both acute circuit resistance and fasting condition in healthy males. These results suggest that plasma nesfatin-1 may have an influential role in short-term anorexia condition and insulin secretion immediately after exercise. Nesfatin-1 secretion is suppressed under fasting condition. Further investigation is warranted with longer exercise duration and a different post exercise follow up on plasma concentrations of this peptide.