1. Background
Growing evidence suggests that sarcosine (N-methylglycine) may be a biomarker indicating the progression of prostate cancer (1). However, the role of serum and urine sarcosine as potential and sensitive biomarkers in prostate cancer progression remains unclear. Recent findings have highlighted the fact that compared to serum prostate-specific antigen (PSA), sarcosine could discover biopsy-positive prostate cancer (PCa) more accurately (2). In addition, recent studies have denoted the possible diagnosis of prostate cancer using sarcosine (3).
Several studies have indicated that the pre-diagnostic power of sarcosine is higher compared to total PSA (4). A direct, positive correlation has also been reported between serum sarcosine concentration and PCa (5). Sarcosine is a derivative of glycine amino acid, which increases in prostate cancer (6, 7). According to the literature, sarcosine belongs to a methyl group and is converted into glycine when catalyzed by sarcosine dehydrogenase (SARDH) (7, 8). According the findings in this regard, sucrose-degrading enzymes SARDH and pipecolate oxidase catalyze the oxidative demethylation of sarcosine, converting it into amino acid glycine (9, 10). However, researchers have reported that plasma sarcosine cannot differentiate between primary conditions and advanced prostate cancer (11).
Previous studies have indicated that PSA has limited specificity and sensitivity due to its increase in men with BPH, prostatitis, and other non-malignancies (12). Nuclear magnetic resonance spectroscopy of the serum in prostate cancer is an invasive method used for the detection of PCa and BPH (13). As previously discussed, the use of sera prostate-specific antigen levels is not sufficient for the differentiation of PCa and BPH and their diagnosis (14). Sarcosine plays a key role in the aggressiveness and progression of prostate cancer (15). Sarcosine is an N-methyl derivate of the amino acid glycine synthesized by glycine-N-methyltransferase (16). However, the biochemical and physiological role of sarcosine is not well-recognized although it is an important intermediary agent in one-carbon metabolism, acting as a one-carbon donor in mitochondrial reactions (17-19).
According to the literature, the serum concentrations of some free amino acids (e.g., sarcosine) are significantly higher in patients with fibromyalgia syndrome compared to healthy controls (20). Moreover, plasma amino acid profiles (e.g., sarcosine) have been reported to be significantly different in patients with aortic dissection and acute atopic dermatitis patients compared to those with coronary heart disease without aortic lesions (21). On the other hand, some researchers have suggested that both sarcosine and prostate cancer move in tandem (22-24), while other findings have reported an inverse process in this regard (2, 25). Due to the discrepancy in this regard, further investigations are required to elucidate the potential role of sarcosine in prostate cancer progression. Moreover, more specific and sensitive biomarkers should be identified, especially for the detection of clinically significant and aggressive prostate cancer (26).
Evidence attests to the elevated urine sarcosine levels in men with prostate cancer (27). Previous studies have also established that serum and urine sarcosine are correlated with the biopsy findings of patients with prostate cancer (28, 29). Additionally, the role of sarcosine in urine for the diagnosis and advanced monitoring of PCa has been investigated (30), and sarcosine metabolism seems to be significantly involved in PCa development (31).
2. Objectives
The present study aimed to investigate the correlations between the circulatory levels of PSA and serum and urine sarcosine levels between patients with BPH and PCa and healthy controls.
3. Methods
3.1. Experimental Materials
All the chemicals were of the highest purity. The human sarcosine ELISA kit (Catalog # MBS720183) was purchased from My BioSource, and the components included enzyme conjugate, sarcosine probe, sarcosine enzyme, and sarcosine standard solution.
3.2. Instruments
The instruments applied in the study were a Labnet Vortex Mixer, centrifuge (Clement 2000, Australia), water bath (Fan Azma Gostar Co., WM22), dionizer (Hastaran Teb Co.), microplate reader (filter: 450 ± 10 nm; Rayto life and analytical sciences, model: RT2100 C, Hamburg, Germany), an ichroma, and a microplate.
3.3. Study Design and Subjects
The serum and urine samples were obtained during 2015 - 2017 from the patients with BPH and NDPCa, as well as the healthy controls enrolled in the annual PSA testing. The blood and urine samples were obtained from each patient early in the morning in the fasting state. In the case of urine samples, sample interferences were eliminated, and the urine samples were collected using sterile tubes and centrifuged at 2,500 rpm for 25 minutes. After the meticulous collection of the supernatants, sample preparation was carried out immediately.
The diagnosis of PCa was based on the criteria of prostate cancer, and none of the subjects received medication. The present study was conducted in accordance with the World Medical Association Declaration of Helsinki. Moreover, the study protocol was approved by the Ethics Committee of Babol University of Medical Sciences in Babol, Iran (code: MUBABOL.HRI.REC.1395.50 Ethic 3319). All the variables (including PSA) were determined using the same serum and urine specimens as those used for sarcosine assessment.
3.4. Serum Specimens
After the routine biochemical check-up, 90 eligible participants were selected and enrolled in the research, and 23 patients were excluded. The sample population consisted of 67 subjects aged 40 - 75 years, who were either diagnosed with prostate cancer and were healthy (controls). It is also notable that the subjects were recruited from the patients referring to Shahid Beheshti Hospital, affiliated to Babol University of Medical Sciences.
Based on the PSA level, 25 subjects with normal PSA history in their previous prostatitis treatment were considered as group one (healthy controls), while group two included the patients with benign prostatic hyperplasia (n = 30) (Table 1). It is also notable that seven patients were excluded due to inadequate quantity for the analysis, and 23 patients remained in the study. The third study group included the patients who were newly diagnosed with prostate cancer (n = 30), and 11 patients were excluded due to inadequate quantity for the analysis. In total, 19 patients remained in the research and further evaluated. On the other hand, the control subjects were delineated as the male patients with the minimum follow-up of two years, reporting no PCa diagnosis. PSA was measured in the same serum sample as those used for serum and urine sarcosine. In addition, data were collected on the age, body mass index (BMI), and disease duration of the patients.
| Variables | Healthy Controls | Patients with Benign Prostatic Hyperplasia | Patients with Prostate Cancer |
|---|---|---|---|
| Number of subjects | 25 | 23 | 19 |
| Mean age, y | 46.9 ± 5.2 | 49.3 ± 9.8 | 63.8 ± 8.6 |
| Gender | Male | Male | Male |
aValues are expressed as mean ± SD.
The inclusion criteria of the study were PSA of ≥ 4 and ≤ 40 ng/dL, BMI of < 30 kg/m2, and presence of prostate hyperplasia/prostate cancer. The exclusion criteria were as follows: (1) patients undergoing chemotherapy/radiation therapy; (2) patients receiving medication; (3) cancer diagnosis; (4) presence of type II and II diabetes mellitus, chronic liver/renal diseases and obesity; (5) smoking habits and (6) history of prostate operations.
3.5. Determination of Sarcosine
Sarcosine levels were measured using the human sarcosine ELISA kit (Catalog # MBS720183), which was purchased from MyBioSource Company.
3.6. Sample Size
The sample size of the research was determined at 95% confidence interval (z = 1.96) and 5% margin of error (d = 0.05).
3.7. Statistical Analysis
Data analysis was performed in SPSS version 16 using descriptive, and the data were expressed as mean and standard deviation. All the tests were repeated in triplicate at the minimum. Comparison of the variables was carried out using student t-test to evaluate the differences between the study groups. In all the statistical analyses, P value of 0.05 was considered significant.
4. Results
According to the obtained results, the patients diagnosed with prostate cancer had abnormal laboratory findings, as well as a positive family history of PCa and elevated PSA (P < 0.001). On the other hand, the healthy control subjects had comparable age and background regarding their previous negative biopsy to the prostate cancer.
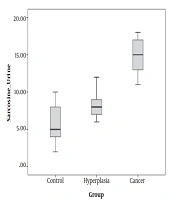
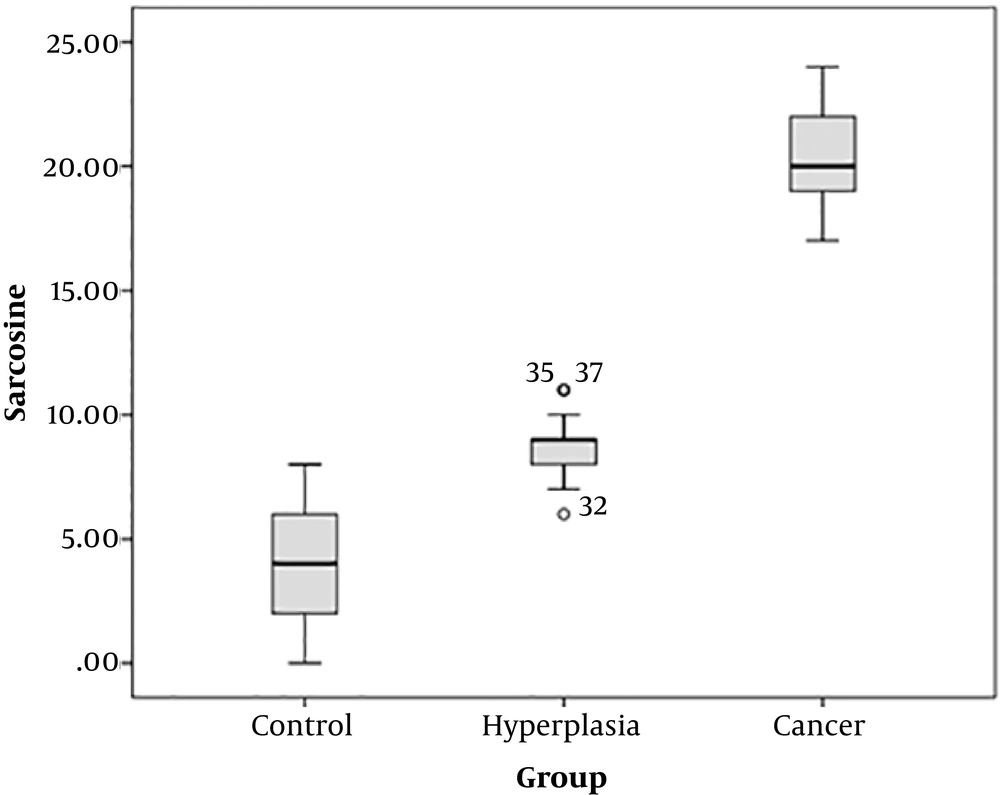
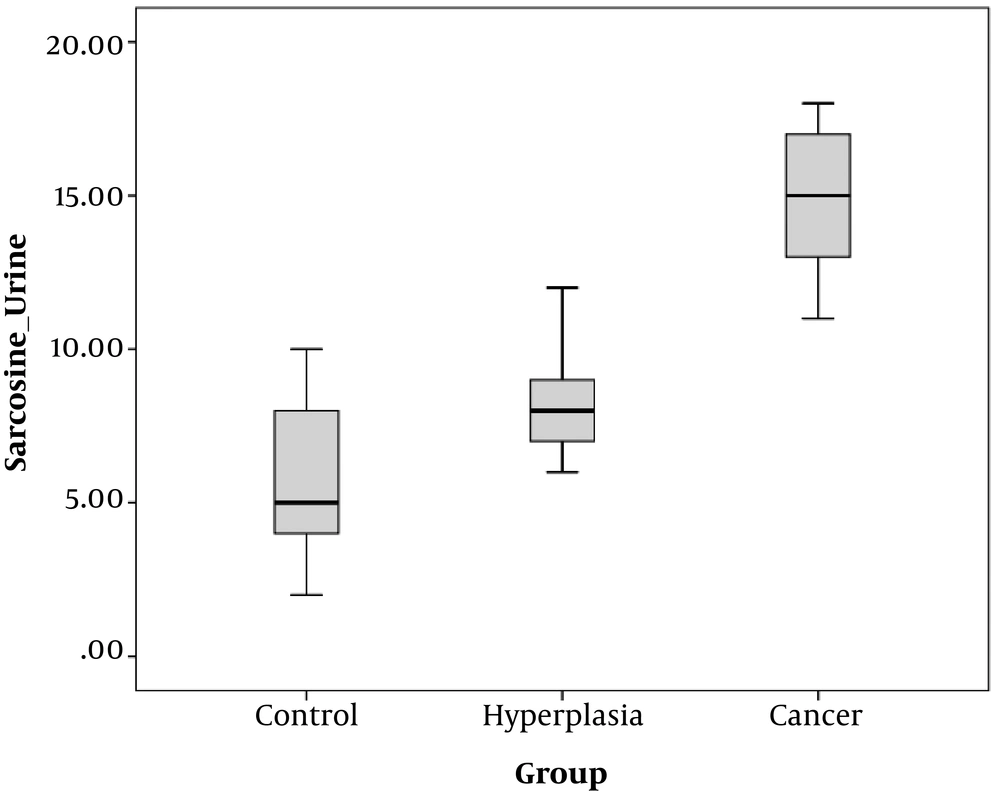
As is depicted in Figures 1 and 2, the mean serum and urine sarcosine levels of the healthy controls were 3.0 ± 2.0 and 6.0 ± 2.0 ng/mL, respectively. In the patients with BPH, the mean serum sarcosine and urine levels were 9.0 ± 1.0 and 8.0 ± 1.0 ng/mL, respectively, while in the patients with NDPCa, these values were estimated to be 21.02 ± ±2.0 and 15.0 ± 2.0 ng/mL, respectively. The serum and urine sarcosine levels were in the following order: healthy controls < patients with BPH < newly diagnosed patients with prostate cancer. It is also notable that the serum sarcosine significantly elevated in the patients with PCa.
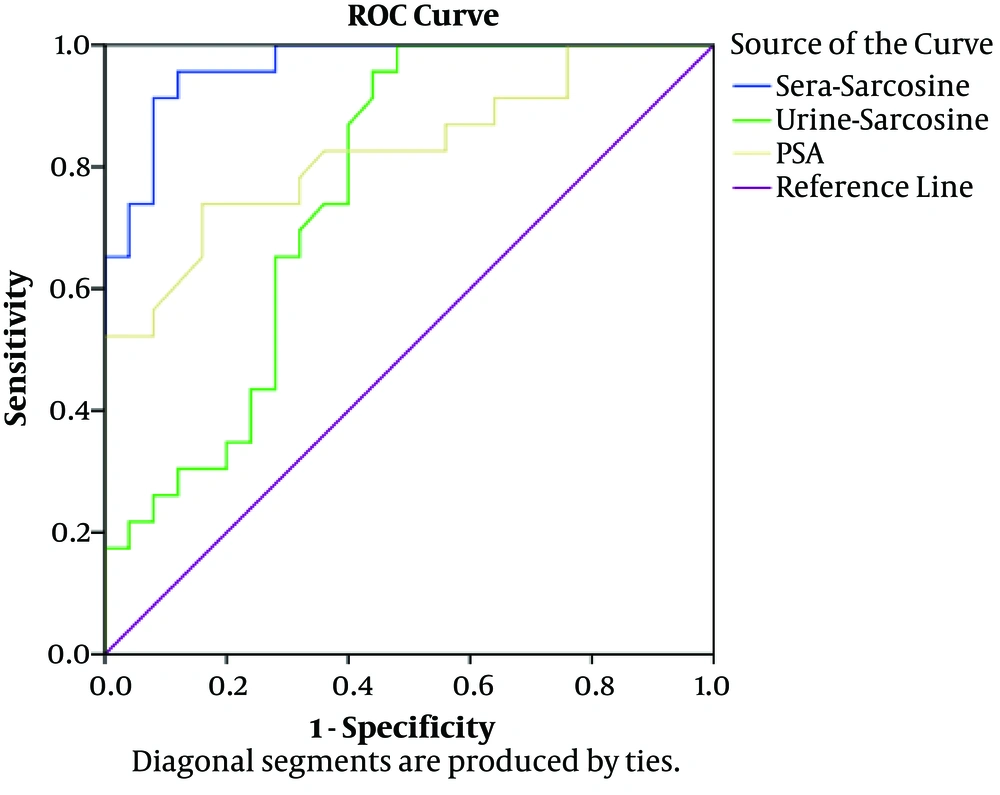
Evaluation of the serum and urine sarcosine levels using the ROC curve analysis demonstrated that the differentiation of NDPCa patients from BPH patients improved based on the sarcosine levels in the serum and urine samples (Figure 3 and Table 2). Furthermore, a direct, positive correlation was denoted between the serum sarcosine content and prostate cancer status. The AUC of PSA and sarcosine in the serum and urine were 0.825, 0.965, and 0.760, respectively, and the independent predictive power of the serum and urine sarcosine could differentiate between the patients with BPH and NDPCa.
aTest result variables: urine sarcosine; PSA with minimum of one tie between positive actual state group and negative actual state group (statistics may be biased).
bUnder nonparametric assumption
cNull hypothesis (true area = 0.5)
5. Discussion
In our ongoing research projects on the human cancer mechanism we under took the present project. In this case–control study the role of serum and urine sarcosine in patients with BPH and NDPCa were investigated. Figures 1 and 2 showing that patients with NDPCa had higher values of serum sarcosine when compared to patients with BPH and healthy controls. Increasing the amount of sarcosine in PCa may be due to increased activity of the enzyme synthesizing sarcosine, or it may be due to decreased activity of it’s degrading enzyme. Therefore, it can be hypothesized that sarcosine metabolism might be impaired in PCa status.
An effective approach to preventing the progression of BPH to PCa is to reduce the levels of sarcosine, and the identification of proper medications to be used in the sarcosine metabolism pathway is considered promising in this regard. The results of the present study indicated positive correlations between the serum and urine sarcosine contents and PSA level in the patients with BPH and NDPCa. As a result, the serum and urine sarcosine levels could differentiate the patients with BPH and NDPCa. These findings are consistent with the distributed data in the urine and sera, highlighting the differences between the controls and patients with PCa in this regard (16).
In 2015, the prognostic performance of sarcosine was investigated in 78 patients undergoing radical prostatectomy for biopsy-proven PCa, and the findings demonstrated that the sarcosine level was significantly higher in the patients with tumors (32). In 2014, a simple and sensitive method was developed for the detection of sarcosine, and sarcosine was reported to be a potential biomarker of PCa (33). Moreover, the obtained results of the mentioned investigation indicated that sarcosine was associated with the increased risk of PCa (34). However, controversies persist regarding role of sarcosine as a biomarker of PCa (33).
The findings of the current research are inconsistent with some studies denoting that sarcosine is not a definitive indicator of PCa in urine samples (11, 14, 30). In a study in this regard, serum sarcosine (N-methylglycine) was assessed in 328 cancer patients using gas chromatography (GC)/mass spectroscopy (MS) in order to determine the correlations with early (stage T1/T2) and advanced diseases (stage T3/T4). The researchers suggested that plasma sarcosine could not differentiate between primary conditions and advanced prostate cancer (11). In addition, sarcosine in the urine specimens of the patients with PCa was not considered to be an important marker of PCa identification (30).
According to the results of the present study, sarcosine is a definitive indicator of PCa when analyzed in the serum and urine samples (2, 4, 35, 36). In another research in this regard, the fluorometric assay was employed for the analysis of the serum samples obtained from 290 PCa patients and 312 patients with no evidence of malignancy, and the findings that were confirmed by 8 - 12 core prostate biopsies suggested a positive correlation between sarcosine concentration and PCa status (4).
According to the literature, urine sarcosine could be utilized along with PSA in various screening protocols (1, 2, 36). However, it is difficult to compare our results with the other studies in this regard due to the variations in the study design, sample populations, sample types, and analytical methods used to measure serum and urine sarcosine. Therefore, further investigations are required to further clarify the role of sarcosine in patients with BPH and NDPCa, thereby contributing to the development of extensive research regarding the mechanism of PCa in order to improve the quality of life of the patients with PCa.
5.1. Limitations of the Study
One of the limitations of the study was the small sample size, which might have decreased the power of the statistical analysis. In addition, data were not available on the disease stage, and further analysis in this regard would have added to the potency of the obtained results.
5.2. Conclusions
According to the results, sarcosine had differential values in the serum and urine samples of the patients with BPH and NDPCa compared to the healthy controls. In general, the acquired data could provide invaluable insight in the function of sarcosine in the differentiation of patients with BPH and NDPCa.