1. Background
Cerebral palsy (CP) is the most common cause of childhood-onset neuromotor disorders, occurring at a rate of approximately 2.1 per 1000 live births, with a higher incidence in low-income countries (1). Environmental and metabolic conditions, infections, hypoxic-ischemic encephalopathy, malformation factors, and genetic disorders are primarily considered causes of CP (1). The clinical presentation of CP is characterized by a non-progressive brain injury or lesion acquired during the antenatal, perinatal, or early postnatal period, leading to limitations in activities (2). CP is commonly classified based on the predominant motor syndrome into spastic hemiplegia, spastic quadriplegia, spastic diplegia, and extrapyramidal or dyskinetic types (3). The spastic CP subtype is present in 70 - 88% of patients with CP. Additionally, spasticity has been reported in approximately 70% of children with dyskinetic CP (4). Spasticity is defined as muscle overactivity with a velocity-dependent increase in tonic stretch reflexes (muscle tone) and exaggerated tendon jerks resulting from hyperexcitability of the stretch reflex and functional deficits (5). Brain injury and impaired development of descending pathways result in limited selective voluntary control and increased tonic muscle activity, which may include spasticity, hypertonia, dystonia, and co-contraction (6). Spastic CP is associated with both neural and non-neural musculoskeletal impairments, including spasticity, decreased selective muscle control, postural instability, muscle contractures, altered intrinsic muscle structure, bony deformities, reduced gross and fine motor function, and pain (7, 8). These abnormalities primarily affect distal muscles, especially in the lower limbs, which can limit the ability to perform daily living activities requiring ambulation. The main functional activities of the lower limbs are directly related to controlling limb movements (9). Several traditional treatments for managing spasticity have been introduced, including rehabilitation, oral medications, chemical neurolysis, rhizotomy, and orthopedic procedures, which may have potential side effects (5, 10). Alternative therapeutic approaches have been found to be easier, more effective, and less painful for patients.
Botulinum toxin type A (BTX-A) is a powerful neurotoxin produced by the anaerobic bacterium Clostridium botulinum, which selectively inhibits the release of acetylcholine at motor endplates, leading to a dose-dependent temporary reduction in tone of the injected muscles (11). BTX-A is considered the first-line treatment for focal spasticity in a limited number of muscles in children with both unilateral and bilateral spasticity. Treatments with BTX-A have also been shown to improve muscle tone, gross motor function, ankle range of motion, and gait speed (12, 13). While numerous studies have highlighted the significant beneficial effects of BTX-A injections, such as reduction in spasticity, improved functional prognosis, delayed and reduced need for surgery, and enhanced quality of life measures (13-16), others have not found such benefits (12, 17).
2. Objectives
Given the ongoing debate both in clinical practice and literature regarding the outcomes of BTX-A treatment on lower limb functional activity in patients with spastic CP, this study aims to examine the impact of BTX-A treatment on lower limb muscle function in children with spastic CP who were unable to complete physiotherapy sessions due to muscle spasms.
3. Methods
3.1. Data Collection and Study Design
This cross-sectional study was conducted from March 2020 to September 2021 at Kowsar Hospital in Semnan, Iran. We reviewed medical records of children aged 2 - 14 years with spastic CP and a minimum spasm severity of +1 on the Ashworth scale, without fixed deformities, who were referred to a plastic surgeon by a physiotherapist due to inability to complete physiotherapy treatment sessions, and who subsequently received BTX-A treatment. We also collected demographic characteristics and types of muscle dysfunction, including knee flexion and extension, heel equinus, and thigh adduction, as determined before BTX-A injection, from their medical records.
The targeted muscles for this study included the gastrocnemius, soleus, and other leg muscles. BTX-A injections were administered at a dose of 5 units/kg across multiple sites (1 unit every 2 cm along the muscle), focusing on areas with the highest concentration of motor endplates in the muscles selected for treatment.
3.2. Assessment of Intervention
Participants underwent evaluations before the injection and one month after the procedure, conducted by a surgeon. Additionally, a comprehensive questionnaire was completed, which included assessments of spasticity using the Ashworth scale, motor performance (such as the ability to walk independently, weight-bearing (WB), and the ability to stand without support), and the satisfaction of parents, surgeons, and physiotherapists with the children's motor performance (rated as low, medium, good, excellent). The questionnaire also inquired about the rate of muscle function improvement, covering aspects like knee flexion, knee hyperextension, thigh adduction, and equinus of the heel (categorized as no improvement, mild improvement, moderate improvement, or big improvement). The study was approved by the Ethical Board of Semnan University of Medical Sciences (IR.SEMUMS.REC.1400.188), and written informed consent was obtained from all participants or their parents.
3.3. Statistical Analysis
For the analysis, the non-parametric Wilcoxon test and the paired t-test were employed for quantitative variables, while Fisher’s exact test was used for qualitative variables. The analyses were conducted using SPSS (version 23, IBM Corp., USA), with a P-value of < 0.05 being considered statistically significant.
4. Results
4.1. Patients’ Demographics
Data from the medical records of 24 children with spastic CP (14 boys and 10 girls) with an average age of 4.50 ± 2.70 years, who were treated with BTX-A and followed for 30 days, were evaluated. Among these, 11 children (45.8%) were aged 3 years or younger, and 13 children (54.2%) were older than 3 years. The number of injections varied, with the majority of children (33.3%) receiving a single dose of BTX-A, while 8.3% received four injections. The remaining children received two or three injections (14 cases, 58.4%). The muscle groups injected in each session included the soleus, adductor, quadriceps, gastrocnemius, and hamstring. The number of affected muscles varied among the children, with 13 children (54.2%) having the involvement of four or more muscles, while the others had three or fewer muscles involved. The most common movement disorders (58.3%) were associated with knee flexion, adduction, and equinus muscle. The demographic and clinical data are summarized in Table 1.
| Variables | No. (%) |
|---|---|
| Sex | |
| Male | 14 (58.3) |
| Female | 10 (41.7) |
| Age, y | |
| ≤ 3 | 11 (45.8) |
| > 3 | 13 (54.2) |
| Number of botulinum toxin-A injections | |
| 1 | 8 (33.3) |
| 2 | 7 (29.2) |
| 3 | 7 (29.2) |
| 4 | 2 (8.3) |
| Number of involved muscles | |
| < 4 | 11 (45.8) |
| ≥ 4 | 13 (54.2) |
| Type of movement disorder | |
| Equinus | 2 (8.3) |
| Equinus and adduction | 1 (4.2) |
| Equinus and knee flexion | 2 (8.3) |
| Equinus and hyperextension | 1 (4.2) |
| Equinus, adduction, and hyperextension | 4 (16.7) |
| Equinus, knee flexion, and adduction | 14 (58.3) |
Demographic and Clinical Characteristics of Patients
4.2. Evaluation of Outcomes
Table 2 presents the mean Ashworth scores, walking, and standing ability variables at baseline and after 30 days of follow-up. The assessment of spasticity severity using the Ashworth scale showed a significant improvement post-BTX-A injection, with the baseline score decreasing from 11.13 to 8.50 after 30 days (P < 0.001). BTX-A treatment significantly enhanced the ability to walk in children with spastic CP, reducing the number of children who were unable to walk from 10 cases before the injection to 2 cases after 30 days, with a mean score decrease of 4.50 (P = 0.005).
| Variables (Mean) | Baseline | Follow-Up | P-Value a |
|---|---|---|---|
| Ashworth score | 11.1 | 8.5 | < 0.001 |
| Walking ability | 0.0 | 4.5 | 0.005 |
| Standing ability | 0.0 | 3.5 | 0.014 |
Analysis of Clinical Assessment Variables at Baseline and Follow-Up Evaluations
Initially, 6 cases were unable to stand, but after the BTX-A injection, all children were able to stand without support, showing an increase in the post-injection score of 3.50 compared to the baseline (P = 0.014).
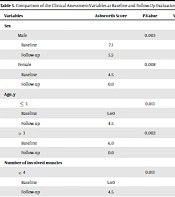
We also evaluated the influence of age, sex, and the number of affected muscles on the outcomes of BTX-A treatment. The results, as shown in Table 3, indicate that BTX-A treatment significantly decreased the severity of spasticity in both boys and girls across all age groups, regardless of the number of muscles involved (P < 0.05). The scores for motor performance variables, including the ability to walk and stand, significantly improved in boys following BTX-A injection (P = 0.008 and P = 0.025, respectively), whereas girls did not exhibit significant improvement (P = 0.317 for both variables). Children aged 3 years or younger showed significant improvements in their ability to walk and stand after BTX-A treatment (P = 0.014 and P = 0.025, respectively), while those older than 3 years did not show significant changes (P = 0.157 and P = 0.317, respectively).
| Variables | Ashworth Score | P-Value | Walking Ability | P-Value | Standing Ability | P-Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 0.003 | 0.008 | 0.025 | |||
| Baseline | 7.1 | 0.0 | 0.0 | |||
| Follow-up | 5.5 | 4.0 | 3.0 | |||
| Female | 0.008 | 0.317 | 0.317 | |||
| Baseline | 4.5 | 4.5 | 0.0 | |||
| Follow-up | 0.0 | 0.0 | 1.0 | |||
| Age, y | ||||||
| ≤ 3 | 0.013 | 0.014 | 0.025 | |||
| Baseline | 5.60 | 0.0 | 0.0 | |||
| Follow-up | 4.5 | 3.5 | 3.0 | |||
| > 3 | 0.002 | 0.157 | 0.317 | |||
| Baseline | 6.0 | 0.0 | 0.0 | |||
| Follow-up | 0.0 | 1.5 | 1.0 | |||
| Number of involved muscles | ||||||
| < 4 | 0.013 | 0.046 | 0.046 | |||
| Baseline | 5.60 | 0.0 | 0.0 | |||
| Follow-up | 4.5 | 2.5 | 2.5 | |||
| ≥ 4 | 0.004 | 0.046 | 0.157 | |||
| Baseline | 5.5 | 0.0 | 0.0 | |||
| Follow-up | 0.0 | 2.5 | 1.5 |
Comparison of the Clinical Assessment Variables at Baseline and Follow-Up Evaluations, Based on Sex, Age and the Number of Muscles Involved
BTX-A injection led to significant enhancements in walking scores for both groups, regardless of the number of involved muscles (P < 0.05). However, improvements in standing scores were only observed in children with involvement of 3 or fewer muscles (P = 0.046), and no improvement was seen in children with 4 or more involved muscles (P = 0.157).
Satisfactory outcomes demonstrated significant improvement in children with spastic CP following BTX-A treatment, with the highest levels of satisfaction from surgeons, physiotherapists, and parents being rated as good and excellent (Table 4). Furthermore, clinically meaningful increases in functional outcomes, including scores for knee flexion, knee hyperextension, and thigh adduction, were observed in children without significant differences related to sex, age, and the number of involved muscles (P > 0.05) (Table 5).
| Satisfaction Score | Surgeon | Physiotherapist | Parent |
|---|---|---|---|
| Low | 2 (8.3) | 0 (0.0) | 0 (0.0) |
| Medium | 8 (33.3) | 4 (16.6) | 7 (29.2) |
| Good | 10 (41.7) | 10 (41.7) | 12 (50.0) |
| Excellent | 4 (16.7) | 10 (41.7) | 5 (20.8) |
Satisfaction Scores of Surgeons, Physiotherapists, and Children Parents Following Botulinum Toxin-A Treatment a
| Variables and Level of Muscle Function Improvement | Values a | χ2 | P-Value |
|---|---|---|---|
| Sex | 3.8 | 0.211 | |
| Male | |||
| Low | 0 (0.0) | ||
| Medium | 8 (57.1) | ||
| Much | 6 (42.9) | ||
| Female | |||
| Low | 2 (20.0) | ||
| Medium | 6 (60.0) | ||
| Much | 2 (20.0) | ||
| Age, y | 1.8 | 0.588 | |
| ≤ 3 | |||
| Low | 0 (0.0) | ||
| Medium | 7 (63.6) | ||
| Much | 4 (36.4) | ||
| > 3 | |||
| Low | 2 (15.4) | ||
| Medium | 7 (53.8) | ||
| Much | 4 (30.8) | ||
| Number of involved muscles | 0.1 | 0.942 | |
| < 4 | |||
| Low | 1 (9.1) | ||
| Medium | 6 (54.5) | ||
| Much | 4 (36.4) | ||
| ≤ 4 | |||
| Low | 1 (7.7) | ||
| Medium | 8 (61.5) | ||
| Much | 4 (30.8) |
Comparison of Final Botulinum Toxin-A Treatment Outcome in Muscle Function Improvement Based on Sex, Age, and the Number of Muscles Involved
5. Discussion
Although the literature has confirmed the effectiveness of BTX-A injections, there is a scarcity of research on its impact on lower limb functional outcomes in children with spastic CP. Spasticity leads to reduced functional capacity and increased inactivity. Various modalities have been introduced to reduce muscle tone, including physical therapy (with a range of motion and stretching exercises), medications (such as oral/intrathecal baclofen, benzodiazepines, clonidine, tizanidine), neurosurgery (like rhizotomies and neural transactions), and orthopedic surgery (involving lengthening, recession, or tendon transfer) (18, 19). However, these treatments are often accompanied by side effects like sedation, confusion, dizziness, vomiting, and central nervous system depression, along with a significant morbidity rate following surgical interventions (19, 20).
This cross-sectional study aimed to assess the effects of BTX-A injections on functional outcomes and spasticity in children with CP one month post-injection. The most frequently observed movement disorders were associated with knee flexion, adduction, and equinus deformities. Additionally, most children received only one BTX-A injection. According to the Ashworth scale, which measures the sum of biomechanical and neural components' interference in passive stretching, injections of BTX-A into the lower limb muscles significantly reduced muscle tone. More crucially, this intervention led to improvements in children's functional outcomes. Nauman et al. examined the effect of BTX-A injections on spastic equinus foot in CP patients and noted a significant decrease in Ashworth scale scores and an increase in the ankle joint's range of motion at the end of the first month (21). Similarly, Camargo et al. observed a marked reduction in the Ashworth scale in CP children with triceps surae spasticity 30 days post-BTX-A injection, with the effects still apparent at day 90 (13).
Focal injections of BTX-A lead to localized muscle weakness by blocking the release of acetylcholine at the neuromuscular junction, resulting in temporary muscle paralysis. BTX-A injections also promote muscle strength and growth, thereby reducing the likelihood of bony deformities due to abnormal muscle pull and contracted tendons and joints (1). Blumetti et al. conducted a systematic review to assess the effectiveness and adverse events of BTX-A compared to other treatments for managing lower limb spasticity in children with CP. They included 31 randomized controlled trials with 1508 participants and, paralleling our results, reported that BTX-A improved gait, joint range of motion, satisfaction, and reduced lower limb spasticity in children with CP (22). Despite our findings on the effectiveness of BTX-A in improving the functional outcomes of children with CP, their study showed contradictory results regarding function (22).
Although one of the primary indications for BTX-A therapy in spastic CP is to diminish muscle overactivity to enhance function in ambulatory children (23), in our study, 8 out of 10 children who were unable to walk at baseline could walk post-BTX-A therapy. Consistent with other research, we noted significant enhancements in the walking and standing abilities of children with spastic CP after BTX-A treatment compared to baseline. Balaban et al. demonstrated that BTX-A injections into the gastrocnemius muscle in children with CP not only reduce spasticity and improve walking patterns but also decrease energy consumption, leading to functional improvements (24). Cosgrove et al., who treated children with CP and severe lower limb spasticity with BTX-A, reported considerable improvements in walking, with significant enhancements in the ambulatory status of all subjects (25). Unlu et al. found significant improvements in standing and sitting scores three and six months after a single multilevel BTX-A injection in children with spastic CP (26).
Regarding the effectiveness of BTX-A, outcomes related to spasticity were evaluated based on sex, age, and the number of involved muscles. While both boys and girls experienced a significant reduction in spasticity, the improvements in walking and standing scores were not significant for girls. This discrepancy in BTX-A efficacy between sexes could be attributed to the generally higher muscle strength and greater physical activity observed in boys compared to girls. We also noted that children of different age groups exhibited a significant decrease in spasticity and improvements in motor performance. However, BTX-A treatment did not enhance the standing ability in children older than 3 years. Aligning with our findings, Fazzi et al. reported improved Gross Motor Function Measure scores for the lower limb in CP children aged 48 months or younger, three months post-BTX-A injection (27). Similarly, Choi et al. indicated that the target muscles for BTX-A injection in CP children varied with gross motor functioning and age, highlighting that younger age at injection and injections in distal muscles were significantly associated with greater improvements in gross motor function (28). Younger children likely retain better functional gains due to a broader potential for development and recovery. Camargo et al. (13) also observed that patients with a higher degree of motor limitation responded less favorably to BTX-A treatment. In contrast, our study demonstrated significant improvements in the severity of spasticity and motor performance after BTX-A treatment, except for standing ability.
A critical factor in assessing the therapeutic success of BTX-A treatment is the satisfaction of children and their families following the procedure. It is essential for parents to have realistic expectations about the outcomes of BTX-A treatment before it begins. Interestingly, we observed high satisfaction levels from surgeons, physiotherapists, and the parents of the children. A study by Seyhan et al. reported similar findings as they evaluated the effects of lower extremity BTX-A combined with physical therapy and rehabilitation (PTR) on children with CP, noting a high level of parental satisfaction with this approach (29). However, Bjornson et al. indicated that parents' expectations for the treatment outcomes of BTX-A in children with CP were not aligned with the actual efficacy of the treatment (30).
Considering that the final outcome of BTX-A treatment varies among individual children, our study provides insights into treatment outcomes, including knee flexion, knee hyperextension, and thigh equinus adduction scores, and shows no significant difference between boys and girls, different ages, and the number of involved muscles. Consistent with our results, Chio et al. found that the Gross Motor Function Measure scores (GMFM-88) for lower limb spasticity significantly increased post-injection in both high- and low-functioning groups of children with CP (28). Additionally, the Adult Spasticity International Registry (ASPIRE) study on Onabotulinum toxin A treatment—a type of BTX-A approved for managing upper and lower limb spasticity globally—reported high levels of clinician- and patient-reported satisfaction and improved overall function in lower limb mobility (31).
This study faced several limitations, including a small number of subjects, the absence of CP subtype classification, the lack of a control or placebo group, and the omission of long-term outcome assessments. Despite these constraints, our findings are significant, demonstrating notable functional improvements following BTX-A treatment for lower limb spasticity in children with CP. Additionally, the moderate to low quality of existing studies, marked by substantial heterogeneity in the range of muscles injected, dosages, and injection techniques, presents a challenge for clinicians formulating treatment plans for children with CP. Consequently, there was a need to evaluate the effect of BTX-A treatment on lower limb muscle function in children with spastic CP, taking into account potential influencing factors. A key innovation of our study, compared to previous research, was the assessment of treatment outcomes with BTX-A in CP children who were unable to complete physiotherapy sessions due to muscle spasms, considering factors such as sex, age, and the number of muscles involved.
In summary, this study demonstrated that BTX-A treatment effectively reduced spasticity and enhanced functional outcomes in children with cerebral palsy, particularly in boys and younger children with fewer muscles involved, and also yielded satisfactory parental and caregiver satisfaction. However, future studies with sufficient power that explore the impact of BTX-A, including comparisons with healthy controls, are necessary to confirm these results.