1. Introduction
An increasing amount of blood glucose causes diabetes mellitus. It is a metabolic disease that has three types (insulin-dependent, insulin-independent, and gestational) and some complications. The number of diabetic patients is rising every year, especially in low- and middle-income countries, as the increasing number of patients in the world was from 108 to 422 million individuals between 1980 and 2014. Additionally, 1.6 million diabetic patients died due to this disease in 2016 (1).
Type 1 diabetes or insulin-dependent diabetes usually occurs in younger individuals. They cannot construct insulin; therefore, for survival, they should use insulin injections. Type 2 diabetes or insulin independence diabetes ordinarily occurs in adult individuals (most patients have this type of diabetes). Obesity and a sedentary life increase the risk of this disease. Patients usually have insulin; however, they cannot use it; therefore, they should treat it with medications. Diabetic patients have some symptoms, such as polydipsia, eating a lot of food because they are usually hungry, and polyuria (2).
Many organs might be disturbed in diabetes. Some complications are neuropathy and foot ulcers, injury of blood vessels of the retina and blindness, loss of function of the kidney, strokes, and heart attacks. Additionally, there is another type of diabetes that occurs in pregnant women named gestational diabetes. Patients have increased blood glucose with a level between the normal and diabetic ranges, and they have a higher risk for diabetes type 2 in the next year (2).
Numerous microorganisms, named microbiota, live in different segments of the digestive system. Additionally, the microbiome implies the microorganisms and their genomes. The gut microbiota of humans is diverse organisms, such as bacteria and yeast. About 100 trillion bacteria inhabit the human gut. Moreover, disturbed healthy diversity of microbiota might cause an illness, named dysbiosis. Dysbiosis has a relation with some diseases like type 2 diabetes, inflammatory bowel disease (IBD) (3), rheumatoid arthritis, autism, obesity, colon cancer (4, 5), and allergy (6). Normal gut microbiota has a role in the immune and nervous system (7), making some vitamins, constructing short-chain fatty acids (SCFAs) that epithelial cells use, and modulating lipid metabolism (2). Firmicutes, Actinobacteria, and Bacteroidetes, respectively, are the most phyla of 8 phyla that exist in the human gut. Moreover, Clostridia, Bacteroidia, Bifidobacteriales, Lactobacillales, and Enterobacterales, respectively, are the most common classes and orders that make gut microbiota in healthy individuals (8).
The above-mentioned bacteria have a relationship with nutrition. A study by Salek Farrokhi et al. investigated the association of gut microbiota and nutrition. For example, there was a positive association between the protein of food, especially herbal proteins, and Bifidobacterium. Furthermore, a positive correlation was observed between Akkermansia and dietary saturated fats. Additionally, a negative association was noticed between this bacterium and total polyunsaturated fatty acids (PUFAs) (5).
The investigation of stool samples showed that we have bacterial and fungal microbiota (named mycobiota). However, the mycobiome had very lower variations than the bacterial microbiome. The high number of mycobiota was from 3 genera, such as Saccharomyces, Malassezia, and Candida, and their species, such as Saccharomyces cerevisiae, Malassezia restricta, and Candida albicans (9). A study about the microbiota of the distal of the esophagus was performed by Pei et al., where Streptococcus, Prevotella, and Veillonella, respectively, were the most common bacteria found in this area. Additionally, Firmicutes, Bacteroides, Proteobacteria, Fusobacteria, Actinobacteria, and TM7 were 6 phyla that were the microbiota of the distal esophagus (10).
Some live microorganisms that improve the activities of the body and regulate normal flora are called probiotics. Additionally, these microorganisms are not digested in the gastrointestinal (GI) tract. Another term is prebiotics, which means some carbohydrates that the GI tract cannot digest and absorb; nevertheless, they have benefits for the microbiota. Moreover, probiotics and prebiotics together make synbiotics. Bacillus subtilis, Bacillus licheniformis, Bifidobacterium lactis V9, Lactobacillus acidophilus ATCC4356, L. rhamnosus GG, L. paracasei Lpc-37, L. plantarum 299v, L. acidophilus NCFM, L. casei, L. fermentum KU200060, and Saccharomyces boulardii are some of bacteria and yeast that are utilized as probiotics in some foods, such as fermented milk. In addition, galactooligosaccharides (GOS), fructooligosaccharides (FOS), lactulose, xylooligosaccharides, red ginseng, arabinoxylans, mannosyl carbohydrates, and beta-glucans have prebiotic effects (11).
Numerous studies revealed the benefits of probiotics, prebiotics, and synbiotics. In a study by Yousefi et al. in which synbiotics were used, a decreased proportion of Firmicutes/Bacteroidetes, inhibition of Escherichia coli and Klebsiella, and enhancement of helpful microbiota, such as Lactobacillus, were observed (12). Additionally, prebiotics had some useful activities, such as antidepressant effects (13). Therefore, a wide range of studies are currently being conducted to look into the impact of probiotics, prebiotics, and synbiotics on disease and medical interventions.
1.1. Pathophysiology of Diabetes
The insufficient production of insulin by B cells is the cause of hyperglycemia in patients with type 1 and type 2 diabetes. In type 2, there is a combination of inadequate B cell majority and function to satisfy the needs of insulin resistance; however, in type 1, B cells are reduced by autoimmune destruction (14, 15).
1.2. Pathophysiology of Type 1 Diabetes
Insulin-producing B cells are severely reduced in type 1 diabetes as a result of autoimmunity, known as insulitis, which destroys pancreatic B cells. Immunological infiltrates include lymphocytes, natural killer (NK) cells, and macrophages (16). Biopsies showed that subjects exhibit atrophy in the tail of the pancreas, where the B cell mass is decreased, and T lymphocytes against B cell epitopes are found (17).
In this type of diabetes, B cell epitopes are internalized by antigen-presenting cells (APCs) that present B cell antigens for major histocompatibility complex (MHC) and MHC class I present them to CD8+ T cells; CD8+ induce B cell apoptosis by perforation. The MHC class II presents B cell antigens to CD4+ T cells, and CD4 increases expression of cytokines, such as interleukin 1, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ). The CD4+ T helper cell 1 plays a very important role in type 1 diabetes; it promotes IFN-γ expression, which causes B cell lysis. CD4+ T helper cell 17 has a minor role in this type; it increases the amounts of interleukin 21 and 17 (18). Autoantibodies against B cells increase the risk of type 1 diabetes. These antibodies are islet-cell autoantibodies (ICA), GAD65, insulin antibodies (IAA), insulin antigen-2 (IA-2), and zinc transporter 8 (ZNT8). Insulin antibody is one of the most sensitive biomarkers for type 1 diabetes (19).
1.3. Type 2 Diabetes
Insulin resistance occurs from genetic and environmental factors and generally affects the skeletal muscle because it internalizes the most glucose. Glucagon, cortisol, growth hormone (GH), and catecholamines are insulin antagonists observed in type 2 diabetes that block insulin physiological actions and cause insulin resistance (20). Endoplasmic reticulum (ER) stress in the liver, pancreatic B cells, and brain has also been implicated in insulin resistance. Reactive oxygen species (ROS) are associated with insulin resistance. Reactive oxygen species accumulation causes lipid peroxidation, protein aggregation, and mitochondrial deoxyribonucleic acid (DNA) damage, all of which result in mitophagy. Because mitophagy decreases mitochondrial count and raises the circulation of free fatty acids, it might indirectly lead to insulin resistance (21).
Weir et al., in their study in 2020, argued that a defective B cell mass is necessary for the development of type 2 diabetes (22). In a study by Yoon et al., 52 adults without diabetes cell mass varied considerably, ranging from 0.25 to 1.5 g. When measured in patients with type 2, there is also an important variation and considerable overlap with non-diabetic controls; however, B cell mass in type 2 diabetes as a group is lower than those without type 2 diabetes (23).
Some studies have shown that numerous individuals with type 2 diabetes have a normal B cell mass due to the overlap between these two groups; however, other researchers contend that this conclusion is false because a patient's B cell mass in type 2 diabetes might fall within the normal range for patients without the disease, although it is abnormal for that particular person (24). According to several autopsy studies, it is indicated that patients with type 2 diabetes who are either lean or have obesity have a reduction of B cell mass within the range of 40 - 60% (23). An inappropriate balance between the rates of B cell birth and death has resulted from the increased demand brought on by insulin resistance over several years. Additionally, the stress of insulin resistance causes the production of senescence and aging markers in B cells (25).
1.4. Old Versus New Strategies for the Treatment of Diabetes
Since there has been a global expansion of diabetes mellitus in the recent century, scientific studies fulfill modern gut microbiota-based strategies for not only treating these patients but also managing other metabolic disorders, including obesity, insulin resistance, and type 2 diabetes mellitus (T2DM) (26).
Impaired glucose homeostasis as a cause of T2DM is due to insulin deficiency and tissue resistance to insulin. Despite the increase in the number of drugs lowering the plasma glucose level in T2DM, there are undesirable side effects, such as significant hypoglycemia, gastric complications, liver toxicity, and body weight gain. For instance, the G protein-coupled receptor 40 (GPR40) or free fatty acid receptor 1 (FFA1) plays an important role in boosting glucose-stimulated insulin secretion (GSIS) on β-cells of the pancreas without the risk of hypoglycemia; therefore, new thiazole-based free fatty acid receptor 1 agonist with decreased lipophilicity yet liver toxicity is taking into account as a new strategy for the treatment of T2DM (27).
Diabetes is often more likely to develop in individuals with aberrant metabolic profiles, such as low insulin, fasting glucose, and hemoglobin A1c (HbA1c). Since patients’ gut microbial composition changes in the situation of diabetes, probiotics can promisingly control glucose, insulin, HbA1c, and other glycemic factors through their influence on gut microflora (28). Probiotics, non-pathogenic live-cell microorganism-based supplements, have physiological, immunological, and biochemical effects on the host body through changes applied to the normal gut microflora, resulting in the treatment of metabolic diseases, especially in obesity and T2DM. The latest studies have revealed that there have been potential mechanisms through which probiotics can administer their positive impacts, such as a decrease in oxidative stress, yet an increase in incretins secretion and adhesion proteins production via intestinal epithelium by further reducing intestinal permeability leading to chronic systemic inflammation and insulin resistance (IR) to be diminished (26).
Despite the wide-ranging discussions on the effects of omega-3 PUFA, one of the most important bioactive lipids, this combination applies some health-positive effects containing GI tissue modifications, serum triglyceride (TG) reduction, and induction of anti-inflammatory effect via alteration in cellular signal pathway. A combination of omega-3 PUFA and probiotic strains is much more effective on IR and obesity. Additionally, the biomass of 14 live probiotic strains, which altogether create probiotic Symbiter, yields a more significant reduction in liver steatosis, hepatic total lipids, TG accumulation, IL-12B p40, IR, and obesity than mono-strains probiotics (26).
A recent clinical trial that was performed by Kobyliak et al. revealed that receiving multi-probiotics and omega-3 PUFA simultaneously once a day for 8 weeks causes more changes in serum lipids and cytokine levels in comparison to consuming single probiotics. As a result, a decrease in chronic systemic inflammation markers, body mass index (BMI), and risk factors for developing T2DM can be noticed, contributing to a better glycemic profile (26).
Two safe yet effective antidiabetic drugs with extensive applications in Asian and Middle Eastern countries, including Iran, are acarbose and repaglinide. Although newer counterparts of these agents are available on the market, they still cannot be replaced. Reaching suitable glycemic levels and better treatment-adherence rates is much more remarkable in taking repaglinide; however, it predisposes the patients to obesity. Acarbose treatment can not only diminish hypoglycemic episodes but is also accompanied by weight loss. Most patients with acceptable fasting plasma glucose (FPG) still have an increased level of Hb1Ac owing to postprandial hyperglycemia complication, which is diabetic cardiovascular morbidity. As 65% of diabetic patients could expire due to atherosclerotic cardiovascular issues, they should be monitored through prandial agents. Despite newer prandial medications, which are not globally available and often expensive, acarbose and repaglinide are cited to be more accessible and cheaper. According to the research on newly diagnosed T2DM patients predisposed to postprandial hyperglycemia, acarbose had a better effect on TG levels but not the same on the drug adherence rate, lowering basic insulin needs and causing gastrointestinal side effects; however, repaglinide had a better function in the drug adherence rate, the ability to reach target PPG (postprandial plasma glucose), and reducing the basic required insulin level; but it can lead to weight gain and hypoglycemia. In general, acarbose is much weaker than its rival, repaglinide, in diminishing blood glucose levels (29).
Ancient substances are currently appealing for significant attention in the treatment of T2DM, including 28 Chinese herbs, the most common of which are Huang Qi, YuZhu, Di Huang, and Shan Zhu Yu, called traditional Chinese medicine (TCM). The known rule for modern medicine, "single compound, single target", is changing to “multi-compounds, multichannel fulfilling treatment of T2DM and even other complex diseases”. Therefore, some destructive effects, such as kidney damage, heart disease, and poor circulation, can be prevented. The constituents of the aforementioned 28 plants are 3-hydroxy xylitol, low molecular weight chitosan oligosaccharide, cortical Moutan polysaccharide-2b, tea polysaccharide, Polygonatum polysaccharide, and quercetin. There are comparable aspects with TCF7L2, IRS1, ENPP1, TNF-α, and PPAR-γ when comparing two networks of human protein-protein interaction and T2DM protein interactions in recent articles. The insulin tyrosine kinase receptor (ITKR) is a precursor of IRS1, which participates in insulin signaling. TCF7L2 regulating T2DM susceptibility is one of the crucial genes (30).
Moreover, there are also newer studies on treating glycolipid-based pre-diabetic T2DM contributing to another herbal formula called San-Ye-Tang-Zhi-Qing (SYTZQ), including Folium Mori, Lotus Leaf, Chinese hawthorn leaf, Salvia miltiorrhiza Bunge, and Paeoniae Radix Rubra. This study has not been extended to human beings, and it has been restricted to rats (31).
According to recent studies on available methods of insulin therapy, two important features that have been attracting great attention is a method that provides an improvement in the ineffectiveness of the old methods. Two prescribed strategies, insulin pump therapy (IPT) and multiple daily injections (MDI) therapy as intensive insulin therapy, used for managing T1D, can reduce microvascular yet macrovascular complications but cannot balance hypoglycemia appropriately. Although IPT has indications of severe hypoglycemia and high A1c, it has not been used since it is not cost-effective and easy to use to date. One of the representatives of Alberta Health, dedicated their budget and medical services to diabetic patients, smoothing the path for these patients to be screened and yielding better self-care. The aforementioned data revealed the importance of classifying studied patients for their better management based on priority. In summary, IPT could be a novel alternative to MDI (32, 33).
1.5. Factors Affecting the Human Gut Microbiota
Different factors affecting the gut microbiota and, as a result, their host include a sedentary lifestyle, certain drugs, hereditary background, epigenetic events, some clinical situations, host-dependent factors, and nutritional habits, and they are elucidated in the following context (26).
Stress, anxiety, pathogens, pollutants, malnutrition, insomnia, vigorous exercise, and other similar factors that put stress on our body affect the gut microbiota and their metabolites by altering the permeability and triggering inflammatory GI problems. Despite the various stressors, the body's biological stress response is the same and related to the hypothalamus-pituitary-adrenal (HPA) axis via releasing catecholamines, glucocorticoids, and other hormones resulting in the regulation of the immune system and GI function by modulating the growth of gut microbiota. As a result, any changes in GI motility and a decrease in digestion alter the substrates for these microbial communities. In another stress response, blood escapes from the GI tract, and this accelerates hypoperfusion and ischemia, in which the degradation of the physical gut barrier occurs and intestinal epithelium permeability increases. Since stress has a great relationship with the immune system, it can lead to alteration in the gut microbiota composition and function (34).
Some drugs present the strongest relationship with the gut microbiota, among which are proton-pump inhibitors (PPIs), metformin, antibiotics, and laxatives. Using PPIs results in an increase in oral bacteria in the gut by altering the gastrointestinal pH. Another effect of PPI use is through inhibiting some commensal gut microbiomes, including Dorea and Ruminococcus. Metformin, one of the most common drugs in type 2 diabetes, leads to an increase in SCFA-producing bacteria. The abundance of Bacteroides species yet Alistipes is shown in individuals utilizing laxatives. Additionally, oral steroid use is associated with a rise in Methanobrevibacter smithii, leading to obesity and increased BMI in human beings (35).
Recent papers have suggested that twins are much more similar in gut microbiome than non-relatives, which supports the key role of genetic and epigenetic incidences. Dynamic epigenetic events affected by nutrients and exercise occur through enzymes, including DNA methyltransferase, DNA hydroxylases, histone acetyltransferases, histone deacetylases, histone methyltransferases, and histone demethylases (36).
It has been shown that some diseases and physiological circumstances present their complications through their impacts on the profile of gut microbiota, including rheumatoid arthritis, IBD, enteric infections, and gestation (37). Studies have shown that the way an infant is given birth reveals the gut microbial profile. In this regard, the infant gut microbiota can differ from the maternal vagina-like microbiota in spontaneous vaginal delivery (SVD) to resembling skin microbiota in the cesarean section (CS). For instance, high levels of Bifidobacterium and Bacteroides are recognizable in the first week of SVD infants; nevertheless, the abundance of Clostridium is observed in CS newborns (37, 38).
One obvious yet astounding aspect influencing the gut microbiota population is age. It is unveiled that the process of aging accompanies an increase in the diversity of the gut microbiota. Infants under 2 or 3 years old are different in their gut microbial community. In other words, the establishment of adult-like gut microbiota takes place at this critical point of age: 2 - 3 first years of life. Furthermore, breastfeeding plays a key role in this invisible organ of human beings: the gut microbiota among neonates (37).
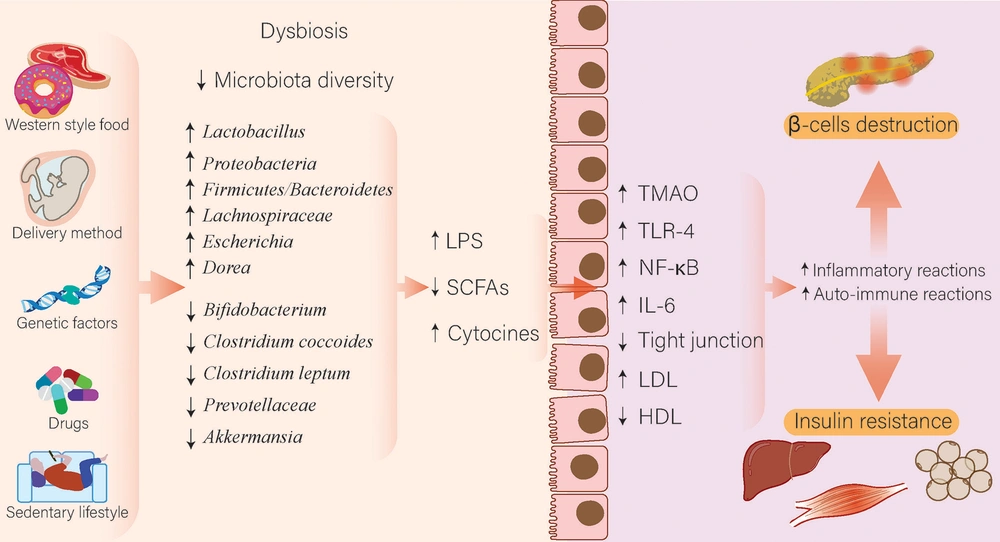
Diet is the main factor providing various nutrients for our nearly 100 trillion intestinal bacteria in the gut. Studies have shown the 1975 Japanese diet mostly included fermented foods, such as fermented soybean foods, resulting in the accumulation of useful bacteria, for example, Lactobacillus and Bifidobacterium, yet decreased harmful bacteria, such as Coliforms and Clostridium. The 1975 Japanese diet is rich in dietary fibers, which allow our intestinal bacteria to produce SCFAs, resulting in host obesity suppression. Furthermore, the intake of this diet provokes changes in four genera of gut microbiome concerning unclassified Lachnospiraceae, Parabacteroides, Sutterella, and unclassified Rikenellaceae, contributing to a decrease in fat%, fat mass, glutamic-oxaloacetic transaminase, (GOT), TG, and HbA1C levels (39). Moreover, low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet and probiotics can regulate microbiota composition and have a beneficial effect on irritable bowel syndrome (IBS) (40). Consumption of fat-enriched food and simple carbohydrate digestion easily evokes changes in the gut microbiome by providing the environment for gram-negative Firmicutes bacteria to rise yet gram-positive Bacteroidetes to decrease. As gram-negative bacteria have lipopolysaccharides (LPS) in the structure of their cell walls, endotoxemia can occur through LPS penetrating the blood due to impaired intestinal permeability. Endotoxemia provokes proinflammatory cytokines, specifically IL-1β, IL-6, and TNF-α, to be overproduced and leads to obesity and glucose metabolism disturbances, in particular resulting in T2DM (26) (Figure 1).
How microbiota affects diabetes. Various conditions, such as delivery methods, western foods, lifestyle, genetic factors, drugs, especially antibiotics and proton pump inhibitors, and a sedentary lifestyle, can disturb the composition of microbiota and cause dysbiosis. In diabetic patients, these changes include an increase in Lactobacillus, Proteobacteria, Lachnospiraceae, and Escherichia spp. and a decrease in Bifidobacterium, Clostridium, Prevotellaceae, and Akkermansia spp. These changes decrease the production of SCFAs and increase the production of LPS and cytokines, which increases inflammatory and autoimmune reactions through various pathways, including TLR-4 and production of interleukin 6, which ultimately leads to increased destruction of pancreatic beta cells and increased insulin resistance of organs.
1.6. Relationship Between Microbiota and Type 2 Diabetes Mellitus
Numerous studies have demonstrated a link between microbiota and type 2 diabetes, with dysbiosis being observed in these individuals. Therefore, the authors would like to highlight a few studies that make this argument. Mansour Sedighi and his team performed a case-control study with 18 diabetic patients and 18 normal individuals. The aforementioned study represented a significant change of composition in the microbiota of patients. It means that in diabetic patients, the quantity of Lactobacillus was increased; however, in healthy individuals, the Bifidobacterium was more. However, the amount of Fusobacterium and Prevotella did not considerably alter (41).
Zhao et al. investigated 65 patients with T2DM and 35 normal individuals. The results showed alteration in gut microbiota and fecal metabolites. The amount of Proteobacteria and the proportion of Firmicutes/Bacteroidetes were increased in T2DM patients. Additionally, patients had a disturbance in bile acids, SCFAs, and lipids. Moreover, Lachnospiraceae and Ruminococcaceae, which construct SCFAs, were raised; nevertheless, the quantity of fecal SCFAs was reduced. Therefore, this finding expressed that some other factors, such as BMI, blood pressure, blood cholesterol, the degree of blood glucose, and fecal bile acids, influence the production of SCFAs (42).
Chen et al. collected samples from 50 patients with T2DM and 50 healthy individuals. They found that some bacteria were different in these two groups. Lactobacillus was higher; however, Clostridium coccoides and Clostridium leptum were reduced in the patients' fecal samples. In addition, the amount of C. coccoides and C. leptum was reduced after treatment of patients for 3 months (43).
A study was designed by Remely et al. on diabetic patients who were under treatment with GLP-1 agonists, obese individuals (these two groups used weight loss matters), and the control group. They collected fecal samples before the use of the GLP-1 agonist and after 4 months of use. The aforementioned study expressed that the proportion of Firmicutes/Bacteroidetes was higher in T2DM patients, and the ratio rose in the second collection of samples. Furthermore, Bacteroides vulgatus, Alistipes spp., Faecalibacterium prausnitzii, Akkermansia muciniphila, and Peptostreptococcus anaerobius had risen between the first collection of samples and their second collection; nevertheless, Bacteroidetes thetaiotaomicron did not alter. Therefore, the gut microbiota was seriously changed in T2DM patients in contrast to obese and normal groups (44).
In a study by Yamaguchi et al., 59 T2DM patients were examined. Additionally, dietary habits and SCFAs were investigated. The study represented an increase in the number of Clostridium clusters IV and XI and a reduction of Bifidobacterium spp., order Lactobacillales, and Clostridium cluster IV in a high intake of carbohydrates, protein, and fats. Moreover, a negative correlation was observed between the protein of diet with fecal acetate and total SCFAs. Moreover, a negative correlation was observed between propionate, acetate, and total SCFAs with the degree of blood insulin (45).
Saeb et al. carried out a case-control study on 15 T2DM patients, 10 impaired glucose tolerance (IGT) individuals, and 19 control subjects. They took saliva samples from the study participants. After the investigation of samples, they observed that the number of species of oral bacteria was decreased in diabetic patients and IGT subjects. Nevertheless, pathogenic bacteria were more common in T2DM patients than in the control group (46).
A study by Yu et al. was about diabetes type 2 in some male mice, which expressed that six grades of Verrucomicrobia (e.g., phylum, class, and order) and family S 24_7 were raised; however, family, genus, and species of Bacteroidaceae besides family and genus of Prevotellaceae reduced. Another finding was the alteration of fasting blood glucose, body weight, fluid, food intake, and diversity of gut bacteria when fecal microbiota was transplanted into pseudo-germ-free mice. Therefore, the aforementioned results showed a relationship between gut microbiota and T2DM. Additionally, using gut bacteria for the treatment of diabetes might be useful (47).
Pushpanathan et al. had a project with 17 T2DM patients and 13 non-diabetic individuals. Diabetic patients had more Escherichia and Prevotella than gram-negative bacteria. However, non-diabetic individuals had more Faecalibacterium, Eubacterium, and Bifidobacterium, which means gram-positive bacteria. Furthermore, in T2DM patients, the mean of monocyte chemoattractant protein-1 (MCP-1) and IFN-γ was increased. Monocyte chemoattractant protein-1 and IFN-γ are cytokines that make inflammatory states and progress diabetes. Moreover, another point was that the LPS of Escherichia could be the reason for low-grade inflammation in T2DM patients (48).
Li et al. designed a study for diabetic patients in the north of China, in which T2DM patients had a lower variation of gut bacteria. Akkermansia and Bifidobacterium that construct butyrate reduced. However, Dorea seriously rose and had a negative association with bacteria that construct butyrate, and it could affect the progression of type 2 diabetes. Researchers observed in this region of China that T2DM is powerfully related to an elevation of butyrate and the metabolism of the gut microbiome that causes some amino acids to deteriorate. The aforementioned study also suggested that the alteration of gut microbiota could help in recognizing individuals who are more at risk for T2DM (49).
A study by Liu et al. represented that individuals who had been exposed to air pollutants for a long time had a reduction in variation of the gut microbiome and an elevation in the risk of T2DM and impaired fasting glucose (IFG). In addition, gut microbiota had a role in the relationship of air pollutants with diabetes risk (50). Therefore, the aforementioned studies and many other kinds of research suggest a relationship between T2DM and the gut microbiome that can be used for treatment and enhancement of the quality of life of diabetic patients (Figure 1).
2. Conclusions
Diabetes is an extremely common disease affecting various age ranges of individuals across the world. It is a serious health issue and has become one of the major diseases that result in death. It can hurt different organs, for example, the cardiovascular system, nervous system, urinary, and kidney. Currently, the prevalence of diabetes is increasing across the world, and it should be given further focus than in the past. We should have the knowledge of diabetes to begin managing the disease early or prevent it. In this review, we have studied some articles about the alteration of the microflora of the GI tract in diabetes and various methods for its treatment, such as using probiotics, prebiotics, and synbiotics. Studies showed that the significant alteration of the microbiota of GI tracts, such as the mouth, esophagus, and gut, occurs in diabetic patients. Pathogenic species and the proportion of Firmicutes/Bacteroidetes rise. Currently, diverse medications, such as repaglinide, acarbose, herbal drugs, and insulin injections, have been used for controlling blood sugar. Additionally, probiotics and prebiotics contribute to enhancing the gut microbiota, and the microbiota has good effects on physiologic mechanisms. Therefore, paying special attention to synbiotics as a new supplement for the treatment of diabetes is necessary, and further studies should be conducted to determine the efficacy of this therapy for patients.